Researchers from one of Europe's leading cancer facilities, the Gustave Roussy Institute in Paris, have attempted to boost understanding of the notoriously difficult subject known as radiomics. They've outlined the five steps needed to validate an imaging biomarker.
Radiomics is an image analysis process that aims to develop imaging biomarkers, and it is based, among other methods, on texture analysis, according to Charly Girot, a doctoral student at the Gustave Roussy Institute, Paris-Saclay University, Villejuif, Paris, and colleagues.
"It considers images not as 'photos' but as a complex dataset," the group explained. "It makes use of large radiological datasets by analyzing them quantitatively using mathematical tools."
Why the fuss about radiomics?
Imaging biomarker development through radiomics is a well-defined process within the international community. Unlike conventional biochemical markers, imaging biomarkers offer real-time access relatively soon after acquisition, are noninvasive, and consider tissues at the macroscopic scale, which makes it possible to take into account heterogeneity.
"It's all about features," the researchers noted. "Radiomics is a multistep process which consists of extracting features. These features come from five major families: shape, filters and transforms, fractal analysis, intensity histogram, and texture."
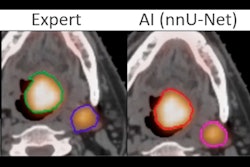
Texture analysis consists of the study of imaging gray-level spatial distribution and is used to quantify image spatial heterogeneity, they stated.
5 steps to validate a biomarker
There are five main steps for biomarker validation.
- Justify the needs for an imaging biomarker. Ask yourself these questions: Can the clinical application benefit from an imaging biomarker? Do radiological data contain relevant information relative to the clinical objective? Do these data exist, and can they be accessed? Can radiomics quantify imaging information? Has this already been done?
- Technical validation. Repeatability, reproducibility bias, and availability are essential to validate robustness so the biomarker can be used by many research groups and in large clinical trials.
- Clinical and biological validation. Correlation to the clinic and the relationship to clinical or biological information are important here. The biomarker should be potentially prognostic, diagnostic, or predictive.
- Regulatory qualification/clearance from the European Medicines Agency, U.S. Food and Drug Administration, and other agencies. Any necessary modifications or improvements must be carried out before the potential biomarker is applied to a large multicentric and prospective study.
- Cost-effectiveness study, comparing costs versus health benefits. Ask these questions: What is the health benefit-risk ratio? What is the examination cost to obtain this biomarker compared with the biological gold standard? Does the added value justify the additional cost?
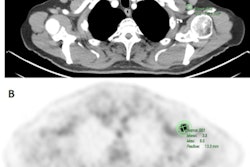
The Paris group uses LIFEx open-access medical image analysis software that provides modules/protocols and supports multiple image formats, including DICOM. The product can be used for PET, MRI, CT, and ultrasound images, is written in Java, and gives access to 42 histograms and textural and shape indices, in addition to conventional indices and where users can change calculation options (e.g., resampling method and number of gray levels for textural analysis).
In PET, the software can be used to extract metabolic tumor volume and total lesion glucose, and it can boost quality control by facilitating PET scanner evaluations using the calibration function. In dynamic contrast-enhanced MRI, the product makes it possible to measure bloodflow, the authors added.
Girot specializes in medical image processing and analysis, and recently he successfully defended his doctoral thesis about evaluation of tumor heterogeneity with parametric imaging. He was the lead author of the e-poster, "Radiomics For Dummies: Image Texture Analysis And Much More...", which received a certificate of merit at RSNA 2021 and forms the basis of this article.