PET imaging combined with a new blood test for Alzheimer's disease may be the best approach to identify people to participate in clinical drug trials, according to a study published on 20 December in JAMA Neurology.
An international group led by Swedish researchers investigated the use of tau PET scans and a blood test for tau phosphorylated at threonine 217 (p-tau-217) for predicting changes in the brain at different clinical stages of Alzheimer disease. The team found that combining the two biomarkers was best for identifying patients in whom the disease was more likely to progress.
"Plasma p-tau-217 and tau PET may significantly reduce sample sizes in preclinical and prodromal [Alzheimer's disease] clinical trials using tau PET as one of the main outcomes," wrote first author Antoine Leuzy, PhD, of Lund University in Malmo.
A widely held view on the development of Alzheimer's disease suggests that beta-amyloid plaque deposits initiate the spread of misfolded tau protein throughout the brain's neocortex and that the accumulation of misfolded tau protein (tau "tangles") in turn causes neurodegeneration.
During the early stages of Alzheimer's disease, patients can show signs of beta amyloid but no obvious symptoms of brain dysfunction. So selecting people for clinical trials of new treatments that can stem the cellular brain changes driving the disease is challenging.
A reliable method for predicting tau tangles in patients who are positive for beta amyloid would have major implications for the selection of clinical trial participants. It would be an important step during the early phases of drug development to avoid large-scale phase III trials with drugs unlikely to result in positive clinical outcomes, the authors wrote.
Recent research has shown that p-tau-217 blood tests can detect abnormal tau metabolism in the brain earlier than PET scans in patients with preclinical Alzheimer's disease. However, no studies yet have addressed which imaging and fluid biomarkers are most strongly associated with abnormal tau at different stages of Alzheimer's disease, the authors wrote.
In this study, the group culled data from an earlier prospective and longitudinal study of Alzheimer's disease performed in 343 individuals in Sweden between September 2017 and November 2020. Beta-amyloid-positive patients who were cognitively unimpaired or who had mild cognitive impairment were enrolled.
The researchers measured baseline tau PET standardized uptake value ratios (SUVR) and annual percent change in tau PET SUVR across five brain regions of interest representing early to later stages of Alzheimer's disease. The blood test detected circulating p-tau-217 levels in the cerebrospinal fluid of patients.
When looking at individual predictors and longitudinal tau PET in the stages that showed most change, the researchers found plasma p-tau-217 (R2 = 0.27, p < .005), tau PET (stage I baseline SUVR; R2 = 0.13, p < 0.05) and amyloid PET (R2 = 0.10, p < 0.05) were significantly associated with longitudinal tau PET in cognitively unimpaired individuals.
In beta-amyloid-positive individuals with mild cognitive impairment, plasma p-tau-217 (R2 = 0.24, p < 0.005) and tau PET (stage II baseline SUVR; R2 = 0.44, p < 0.001) were also significantly associated with longitudinal tau PET.
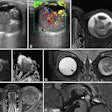
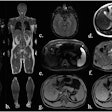
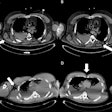
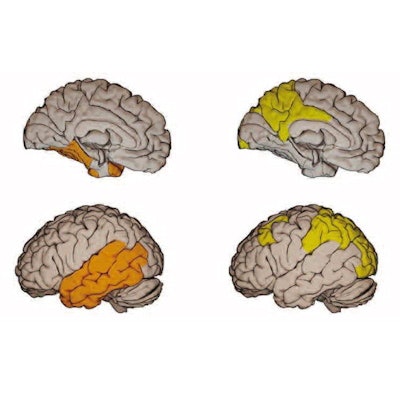
 Surface-based rendering of event-based modeling (EBM) tau PET stages (I through V, left to right). Image courtesy of JAMA Neurology.
Surface-based rendering of event-based modeling (EBM) tau PET stages (I through V, left to right). Image courtesy of JAMA Neurology.Next, having established which biomarkers best predicted tau accumulation in beta-amyloid-positive cognitively unimpaired patients and those with mild cognitive impairment, the researchers performed a power analysis to calculate whether the tests could narrow the field for including patients in a clinical trial.
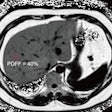
They found plasma p-tau-217 in combination with tau PET could result in patient sample size reductions of 43% (p < 0.005) in beta-amyloid-positive cognitively unimpaired individuals and 68% (p < 0.001) in beta-amyloid-positive individuals with mild cognitive impairment.
"Although our findings suggest that plasma p-tau-217and tau PET could be used as straightforward approaches to identify [cognitively unimpaired] individuals and those with [mild cognitive impairment], respectively, who are more likely to have high rates of tau accumulation, the greater sample size reductions seen when combining plasma p-tau217 with tau PET suggest that this approach may be favorable," the researchers wrote.
The approach could be cost-effective in trials involving preclinical and prodromal Alzheimer's disease, given that a baseline tau PET scans would anyway be required if using longitudinal tau PET as an endpoint and that plasma p-tau-217 is comparatively easy and inexpensive to measure, they added.
Further work addressing optimal biomarker combinations is required, yet the results of this study indicate that plasma p-tau-217 and tau PET may significantly reduce sample sizes in preclinical and prodromal AD clinical trials designed to detect either target engagement or clinical efficacy of new treatments, the group concluded.