A new PET tracer proved for the first time it can be used to identify brain activity involved in the formation of plaque in people with cognitive impairment and Alzheimer's disease, according to a study published on 15 July in Molecular Psychiatry.
The researchers studied the uptake of carbon-11 (C-11) BU99008 in diseased brain regions of patients with late-life cognitive impairment compared with healthy patients. They found direct evidence that the tracer can identify activity involved in the development of amyloid plaque, a hallmark biomarker of neurodegeneration.
The study advances efforts to understand the pathology involved in the formation of amyloid plaque in Alzheimer's disease and other neurodegenerative conditions and could lead to earlier treatments.
"We believe that 11C-BU99008 is a potential new tracer for assessment of the trajectory of in vivo astrocyte reactivity with Alzheimer's and other late-life neurodegenerative diseases," wrote Dr. Paul Edison of Imperial College London and colleagues.
Astrocyte reactivity is the overproliferation of glial cells called astrocytes due to damage of nearby neurons from trauma, infection, or neurodegeneration, for instance. Studies suggest reactive astrogliosis may precede early pathological signs of Alzheimer's disease, including the formation of amyloid plaque and tau tangles.
By identifying astrocytes with PET tracers, the researchers hope to visualize their involvement in the development of the neurodegenerative disease.
Edison and colleagues have been studying C-11 BU99008 since it was synthesized in 2012. In April, a separate group of Swiss researchers published the first results showing the tracer was effective in human Alzheimer's disease tissue samples.
In this study, the group from Imperial College London tested for the first time whether the uptake of C-11 BU99008 is increased in older cognitively impaired patients compared with healthy people of the same age.
Eleven patients clinically diagnosed with probable dementia or mild cognitive impairment due to Alzheimer's disease were recruited and matched against nine healthy patients. Beta-amyloid plaque was identified in patients on PET imaging with F-18 florbetaben (Neuraceq), a tracer approved for that purpose in the U.S. in 2014. C-11 BU99008 was synthesized onsite and imaging was performed using a PET/CT scanner (Biograph TruePoint, Siemens Healthineers).
The researchers found C-11 BU99008 uptake was higher in eight beta amyloid-positive patients with cognitive impairment across the whole brain, but particularly in frontal, temporal, medial temporal, and occipital lobes, compared with the control group. Global tracer uptake (mean uptake ± standard deviation) was 82.7 for cognitively impaired subjects and 77.7 in healthy patients.
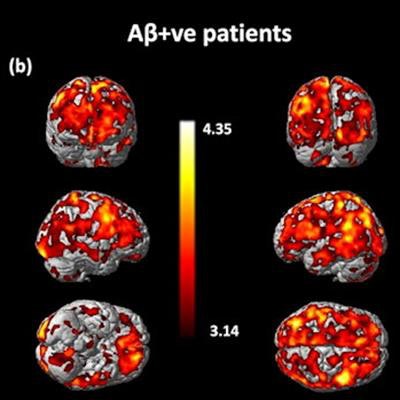
 Statistical parametric mapping analysis of significantly increased C-11 BU99008 uptake as a group for (a) all cognitively impaired (CI) subjects and (b) beta amyloid-positive CI subjects compared with healthy controls, using a cluster threshold of p < 0.05 and with extent threshold of 50 voxels. The color bar indicates the Z-score. Image courtesy of Molecular Psychiatry.
Statistical parametric mapping analysis of significantly increased C-11 BU99008 uptake as a group for (a) all cognitively impaired (CI) subjects and (b) beta amyloid-positive CI subjects compared with healthy controls, using a cluster threshold of p < 0.05 and with extent threshold of 50 voxels. The color bar indicates the Z-score. Image courtesy of Molecular Psychiatry."This proof-of-concept study provides direct evidence that C-11 BU99008 can measure in vivo astrocyte reactivity in people with late-life cognitive impairment and Alzheimer's disease. Our results confirm that increased astrocyte reactivity is found particularly in cortical regions with high beta-amyloid load," the researchers wrote.
While this was only a pilot study, it suggests the potential for using C-11 BU99008 for longitudinal studies of relationships between astrocyte reactivity and other neuropathological and clinical features in vivo and for exploring the impact of therapeutic approaches targeting astrocyte reactivity, the researchers wrote.
"Future studies now can explore how clinical expression of disease varies with astrocyte reactivity," Edison et al concluded.