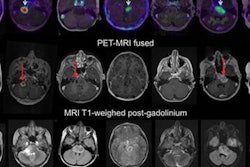
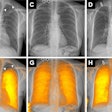
Researchers are one step closer to proving the worth of zirconium-89 (Zr-89) immuno-PET as a noninvasive approach to predicting the effectiveness of therapy for cancerous tumors, according to a study published in the December issue of the Journal of Nuclear Medicine.
The proof-of-concept results show that Zr-89 immuno-PET can be used to measure monoclonal antibody concentrations in normal tissues and, by doing so, potentially lead to more personalized, precision treatment for patients.
"This study shows that nonspecific uptake of monoclonal antibodies for tissues without target expression can be quantified using Zr-89 immuno-PET at multiple time points," wrote the authors, led by Dr. Yvonne Jauw from the Cancer Center Amsterdam, Amsterdam University Medical Center (JNM, December 2019, Vol. 60:12, pp. 1825-1832). "These results form a crucial base for measurement of target engagement by therapeutic antibodies in vivo with Zr-89 immuno-PET."
Currently, the only ways to determine and predict the efficacy of therapeutic antibodies in treating tumors are through invasive tissue sampling or blood samples. While it is known that whole-body Zr-89 immuno-PET can measure the activity of therapeutic antibodies through standardized uptake values (SUVs), it is still unclear which patients are most likely to benefit from treatment and how best to administer it.
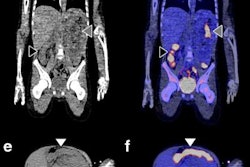
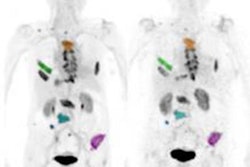
For this retrospective analysis, the researchers collected data from 36 patients who underwent a total of 128 PET scans of the kidneys, liver, lung, and spleen at Amsterdam University Medical Center; Centre Hospitalier Régional at the Universitaire de Lille in Lille, France; and Memorial Sloan Kettering Cancer Center in New York City. The exams were conducted over one to seven days after injection with an appropriate Zr-89-labeled antibody to image their tumors. The four Zr-89-labeled antibodies were Zr-89 obinutuzumab, Zr-89 cetuximab, Zr-89 huJ591, and Zr-89 trastuzumab. Nonspecific uptake was defined as activity measured in tissues without known target expression, which is to say, normal tissue.
Jauw and colleagues were able to quantify nonspecific uptake of monoclonal antibodies in normal tissue using Zr-89 immuno-PET at multiple time points during the seven-day period. By having this data from antibody distribution to normal tissues and tumors, the researchers believe they can enhance their knowledge of which drugs will be effective and, conversely, which drugs are likely to cause toxicity in cancer patients.
"Usually, Zr-89 immuno-PET scans are analyzed at a single time point, representing the sum of all physiologic components of antibody distribution, being either target-specific or nonspecific," the researchers explained. "This study showed how the various physiologic components of antibody distribution contribute to the measured SUV."
In addition, determining the activity of monoclonal antibodies in normal tissue can help researchers discern which monoclonal antibodies are worthy of further development as a therapeutic antibody, Jauw and colleagues wrote. The absence of uptake by a therapeutic antibody could be an indication that it will not be toxic to patients.
"For future studies, a pilot phase including at least three scans at one or more days after injection is required to assess nonspecific uptake as a function of time and optimize study design for detection of target engagement," the researchers added.