Additional training in FDG-PET/CT to monitor concurrent chemotherapy for patients with stage III non-small cell lung cancer (NSCLC) can significantly extend both overall and progression-free survival, according to an international study published in the October issue of European Journal of Nuclear Medicine and Molecular Imaging.
The findings come from the PERTAIN trial, which targeted low- and middle-income countries to assess the efficacy of FDG-PET/CT guidance during cancer treatment. One important key to success was collaborative training to improve interobserver interpretations and consensus among radiation oncologists and also nuclear medicine physicians who are less skilled in using the hybrid modality.
"The PERTAIN trial improved or reaffirmed collaborative working relationships between nuclear medicine and radiation oncology departments in the participating centers," wrote the study authors, led by Tom Konert, a clinical field engineer and doctoral candidate from the Netherlands Cancer Institute in Amsterdam (EJNMMI, October 2019, Vol. 46:11, pp. 2235-2243). "This collaboration may not only have led to improved [tumor] delineation but also may have improved patient management by streamlining the patient pathway from diagnosis to treatment."
Choosing treatment
FDG-PET/CT has long been the modality of choice to stage patients with non-small cell lung cancer and to help chart their treatment course. For stage III NSCLC patients, for example, chemotherapy currently is the recommended regimen. The hybrid modality also has been used for radiotherapy target volume delineation, which is a critical step in determining the most appropriate cancer treatment based on several characteristics that include gross tumor volume, clinical target volume, and planning target volume.
"PET is specifically helpful in target volume delineation when the tumor is not easily distinguished from surrounding healthy tissue on CT images, due to its higher soft-tissue contrast," the authors wrote. "Even with the use of PET imaging, there is still interobserver variability due to differences in the target volume delineation method and FDG uptake in normal structures adjacent to the tumor."
To help remedy that issue, the International Atomic Energy Agency (IAEA) in 2015 established a set of guidelines for the use of FDG-PET/CT in radiation therapy planning for NSCLC patients. One goal was to improve target volume delineation accuracy and reproducibility in imaging centers in low- and middle-income countries. The initiative included training for radiation oncologists and nuclear medicine physicians to improve interobserver agreement on hybrid image results.
PERTAIN study
To assess the progress of the campaign, the IAEA coordinated the design of the PERTAIN study. Participants included nine medical centers in seven countries: Brazil, India, Jordan, Pakistan, Turkey, Vietnam, and Estonia. The project included hands-on training for nine teams, which paired a radiation oncologist and a nuclear medicine physician with limited experience in PET/CT-based target volume delineation to learn how to approach patients with NSCLC based on IAEA protocols.
For the trial, researchers retrospectively collected data from 230 stage III NSCLC patients who were treated at the facilities between January 2010 and July 2014 and had a median follow-up time of 14 months. Also, there was a prospective group of 69 stage III NSCLC patients who were treated between August 2015 and October 2018 and were followed for at last one year.
All 299 subjects underwent whole-body FDG-PET/CT imaging on a number of different systems (Discovery ST, Discovery 710, Discovery STE, GE Healthcare; Biograph 40 mCT and Biograph 64 mCT, Siemens Healthineers). FDG dose ranged from 226 MBq to 441 MBq, with scans beginning approximately 60 minutes after administration.
In the retrospective NSCLC group, 147 patients (64%) received concurrent chemoradiotherapy, 65 patients (28%) underwent sequential chemoradiotherapy, and 18 patients (8%) were given radiotherapy only. All 69 subjects in the prospective group received concurrent curative chemoradiotherapy. In that cohort, 29 patients (42%) also underwent intensity-modulated radiotherapy (IMRT), and two patients (3%) had volumetric modulated arc therapy (VMAT).
Survival rates
"The primary end point was overall survival, defined as the time between the start of treatment and date of death or loss to follow-up," the authors wrote. "The secondary end point was progression-free survival ... defined as the time from the start of treatment to local failure, time to regional failure, and/or time to distant failure."
Overall survival was significantly longer in the prospective NSCLC patient group than in the retrospective cohort (p = 0.012), the team found. The finding was the same for median progression-free survival, which was significantly longer in the prospective group, compared with the retrospective NSCLC patients (11 months) (p = 0.012).
| Survival outcomes for NSCLC patients | |||
| Prospective group | Retrospective group | p-value* | |
| Overall survival | 23 months | 24 months | 0.012 |
| Progression-free survival | 17 months | 11 months | 0.012 |
The significantly better outcomes in the prospective group suggest a "benefit from implementing FDG-PET/CT-guided concurrent chemoradiotherapy in patients with stage III NSCLC in centers in low- and middle-income countries with limited experience with PET/CT," Kornet and colleagues wrote. The results also showed how training in tumor volume delineation under IAEA study guidelines were implemented successfully and that participating facilities remained in compliance.
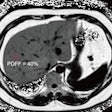
The researchers, however, did not discount other potential factors for the improved overall survival and progression-free survival numbers. All 69 patients (100%) in the prospective group received concurrent chemoradiotherapy with 42% of them also undergoing IMRT. Only 64% of patients in the retrospective cohort received concurrent chemoradiotherapy, which could have affected survival results.
Even with those caveats, the researchers concluded the "initial analysis of the PERTAIN study showed that a combined package of FDG-PET/CT-planned radiotherapy, the routine use of concurrent chemoradiotherapy with training support, and a robust quality control process led to improved overall survival and progression-free survival in patients with stage III NSCLC patients in low- and middle-income countries."