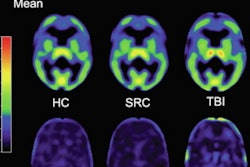
A research team from the U.K. and Sweden has used dynamic flortaucipir-PET imaging to show that single moderate-to-severe traumatic brain injury can trigger signs of accumulation of neurodegenerative tau protein and lead to cognitive decline.
The researchers found significant tau buildup in a brain region associated with object recognition, and they observed a correlation between the increased deposits that reduced cognitive abilities among patients with a more severe TBI almost 20 years after their injury (Science Transitional Medicine, 4 September 2019).
"The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI," wrote the study authors, led by Dr. Nikos Gorgoraptis, from the department of brain sciences at Imperial College London in the U.K. "It is also likely to assist in patient selection and stratification for future treatment trials targeting tau."
Flortaucipir (Avid Radiopharmaceuticals) has recently shown promise in binding to tau neurofibrillary tangles in postmortem brain tissue of Alzheimer's patients. It has also been shown to build up in brain regions associated with chronic traumatic encephalopathy (CTE) among former professional football players who experience multiple head traumas.
Tau deposition also is known to occur after one TBI, with "abundant and widely distributed neurofibrillary tangles [having] been found postmortem in about one-third of patients with TBI," Gorgoraptis and colleagues noted.
What remains unexplored is flortaucipir-PET's role in quantifying tau accumulation many years after a single TBI, and how much that injury might affect a patient's health?
To answer the question, the researchers looked at 21 patients (median age, 49 years; range, 29-72 years) with one moderate-to-severe TBI. Examinations of their injuries, primarily from traffic accidents (86%), occurred at a median age of 32 years (range, 18-51 years). For comparison purposes, 11 age- and demographically matched healthy controls were included in the study.
All participants underwent dynamic PET imaging for 90 minutes with an average intravenous bolus (250 MBq) of flortaucipir, as well as structural 3-tesla diffusion-tensor MR imaging (DTI-MRI) to assess white-matter integrity through fractional anisotropy.
Additional assessments included the Glasgow Outcome Scale-Extended (GOS-E) index, which measures brain function after an injury on a scale of 1 (brain dead) to 8 (good recovery), and a Mini-Mental State Examination (MMSE). An MMSE score of less than 12 is considered severe dementia, while a score greater than 24 is normal.
The researchers then divided TBI patients into two groups: a "disabled" subgroup of subjects with a GOS-E index of 6 or less and a "good recovery" subgroup with an index greater than 6. Longitudinal data was available for 15 TBI patients beginning at a mean of 17 years before the current clinical assessments.
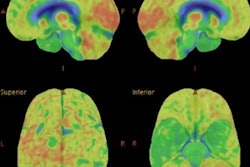
Flortaucipir-PET images showed significantly greater tracer binding to tau deposits in the right lateral occipital cortex (p < 0.05) of TBI patients, compared with the healthy controls. In addition, white-matter integrity, as measured by fractional anisotropy, was significantly reduced in TBI patients (p = 0.04), compared with healthy controls.
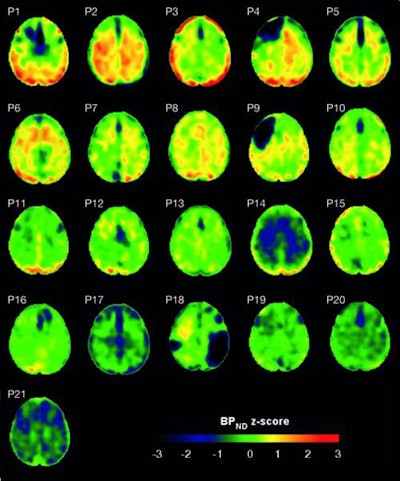 Flortaucipir nondisplaceable binding potential (BPND) z-score maps for each TBI patient are compared by voxel to healthy controls. TBI subjects are presented in descending order by number of voxels with flortaucipir BPND (z > 1.645). Patchy cortical and subcortical increase in tracer uptake is observed in some TBI participants, most consistently in the lateral occipital cortex (P1 to P8 and P10), while other TBI maps show similar BPND values as healthy control subjects (P17 to P21). Images courtesy of Gorgoraptis et al and Science Transitional Medicine.
Flortaucipir nondisplaceable binding potential (BPND) z-score maps for each TBI patient are compared by voxel to healthy controls. TBI subjects are presented in descending order by number of voxels with flortaucipir BPND (z > 1.645). Patchy cortical and subcortical increase in tracer uptake is observed in some TBI participants, most consistently in the lateral occipital cortex (P1 to P8 and P10), while other TBI maps show similar BPND values as healthy control subjects (P17 to P21). Images courtesy of Gorgoraptis et al and Science Transitional Medicine.The researchers also found evidence of a cognitive decline in MMSE scores (average, - 1.3; - 0.073 MMSE points per year) in the disabled TBI subgroup, compared with the good outcome TBI subgroup, which actually improved its collective MMSE scores (average, 1; 0.058 MMSE points per year), creating a statistically significant difference between the two subgroups (p = 0.041).
Given flortaucipr-PET's ability to detect tau in patients after a single TBI, Gorgoraptis and colleagues suggested the technique could help in the design of future trials of tau-targeting therapies.