Almost one-third of breast cancers detected in a high-risk screening program using breast MRI were already visible at the last negative MRI scan, Dutch researchers have reported. The results apply to both screen-detected and interval cancers, they added.
The study team paired data from an increased-risk breast cancer screening program and the Dutch national cancer registry. Dr. Suzan Vreemann, formerly of the radiology and nuclear medicine department at Radboud University Medical Center in Nijmegen, the Netherlands, and colleagues found that 31% of cancers were visible at the initially negative MRI scan.
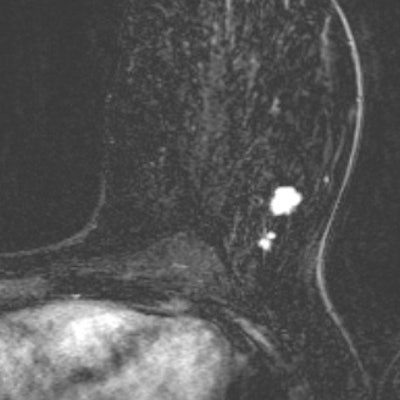
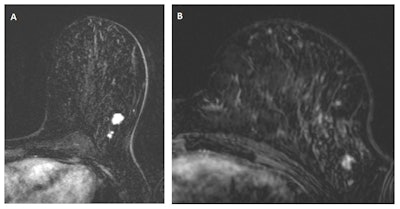 In "A," a multifocal tumor is detected in the left breast of a 49-year-old woman. "B" depicts an MRI scan from the same breast, one year earlier. Despite the lower image quality and the difference in positioning (different scanner and coil), the cancer is clearly visible and should have been recalled according to the consensus read by the radiologists. Images courtesy of Drs. Suzan Vreemann and Ritse Mann.
In "A," a multifocal tumor is detected in the left breast of a 49-year-old woman. "B" depicts an MRI scan from the same breast, one year earlier. Despite the lower image quality and the difference in positioning (different scanner and coil), the cancer is clearly visible and should have been recalled according to the consensus read by the radiologists. Images courtesy of Drs. Suzan Vreemann and Ritse Mann."While we expected that a substantial number of cancers would already be visible in prior scans, due to well-documented numbers on the frequency of visible findings in prior mammography screening exams (about 25% after double reading), we were surprised by the magnitude of this problem," Vreemann and corresponding author Dr. Ritse Mann, also from the radiology and nuclear medicine department at Radboud University Medical Center, told AuntMinnieEurope.com.
Missing cancers
Women with more than a 20% lifetime risk of developing breast cancer are invited for intensified screening programs including both mammography and breast MRI, because the evidence is clear that MRI is more sensitive than mammography. Researchers regularly audit mammography screening programs, but what about the programs that use breast MRI?
The American College of Radiology (ACR) released guidelines on this topic, but they do not include the evaluation of prior screening rounds in women who present with breast cancer.
"To our knowledge, no studies report on the visibility of interval cancers, tumors detected on mammography, or tumors in prophylactic mastectomy specimen in the prior MRI scans," the authors wrote (Breast Cancer Research and Treatment, 31 January 2018).
Thus, in their study, they sought to evaluate the frequency of visible breast cancers detected in women participating in a high-risk screening program in the Netherlands that were missed on a prior MRI scan. They included screen-detected cancers, interval cancers, and cancers in prophylactic mastectomy specimens to assess patient and imaging factors contributing to the lack of detection.
The researchers pulled patient files from 2003 to 2014 for women participating in an increased-risk mammography and MRI screening program. They compared those with the Dutch national cancer registry and determined whether an MRI scan was available zero to 24 months before cancer detection that was reported to be negative. Two dedicated breast radiologists re-evaluated in consensus the negative MRI scans with knowledge of the cancer location. Cancers were scored as invisible, minimal sign, or visible. Additionally, BI-RADS scores, background parenchymal enhancement, and image quality (perfect, sufficient, or bad) were determined.
The study team found negative prior MRI scans for 131 breast cancers. Of these, 76 cancers were MRI-detected, 13 were mammography-detected, 16 were interval cancers, and 26 were incidental cancers. Overall, 31% of cancers were visible at the initially negative MRI scan.
| Lesion visibility on prior negative MRI scans in the Netherlands | ||||
| N (%) | Invisible (%) | Minimal sign (%) | Visible (%) | |
| Overall | 131 (100) | 45 (34) | 45 (34) | 41 (31) |
| BRCA | 70 (53) | 34 (49) | 23 (33) | 13 (19) |
| Non-BRCA | 61 (47) | 11 (18) | 22 (36) | 28 (46) |
| Minimal BPE* | 83 (63) | 30 (36) | 27 (33) | 26 (31) |
| Mild BPE | 22 (17) | 5 (23) | 9 (41) | 8 (36) |
| Moderate BPE | 12 (9) | 3 (25) | 4 (33) | 5 (42) |
| Marked BPE | 14 (11) | 7 (50) | 5 (36) | 2 (14) |
| Perfect image quality | 111 (85) | 43 (39) | 34 (31) | 34 (31) |
| Sufficient image quality | 18 (14) | 1 (6) | 10 (56) | 7 (39) |
| Bad image quality | 2 (2) | 1 (50) | 1 (50) | 0 (0) |
The findings also show that image quality plays a role in the visibility of findings -- the poorer the image, the greater the chance of missing something. If the image is inferior in quality, consider rescanning the patient, the authors wrote.
In terms of BI-RADS, 29% of the 131 lesions were rated as BI-RADS 4 or 5 and, thus, should have been recalled.
"When including cancers presenting with minimal signs, 65% of the lesions were already recognizable on the prior MRI exam," the authors noted. "Both from a learning perspective and in terms of liability, it is essential that these figures are available."
These results are in line with previous studies using smaller cohorts that found 56% and 47% of breast cancers were already visible on prior MRI examinations and retrospectively assessed as BI-RADS 3 or higher. However, previous studies only included screen-detected cancers on MRI.
"To our knowledge, this study is the first to show that also 31% of interval cancers and even 35% of incidental cancers can be identified at the last negative MRI scan," Vreemann and colleagues wrote.
And because the Dutch screening program sensitivity is 89.7%, which is comparable to Italian and German studies, it is likely the new findings are applicable to all breast MRI screening settings, they added.
"It should be noted that this is clinical reality," Vreemann and Mann wrote in their email. "We couldn't pinpoint the missed carcinomas to any specific radiologist. Everyone seems to make these kinds of errors. We do not advocate the use of a disclaimer on every radiology report, but for jurisdiction it should be noted that such errors are normal and you can't blame a single radiologist for a single error, even in retrospectively obvious cases."
What can be done?
The consequences of missing cancer in an MRI scan obtained before prophylactic mastectomy are relatively minor because it's unlikely that these missing lesions will alter the patients' prognosis. However, the consequences of missing breast cancers that present subsequently as interval cancer are dire -- these cancers usually are invasive, poorly differentiated, and fast-growing.
"Detection of these cancers at the MRI examinations could still have had a significant effect on subsequent prognosis and warrants investigation of methods to reduce false-negative reporting of MRI scans," the authors wrote.
Provided that some cancers are missed due to interpretation errors, decreasing the threshold for recall could reduce the frequency of missed cancers, they added. However, the rate of false-positive findings differs depending on the clinical indication for screening; it is relatively low in women with BRCA mutations or a personal history of breast cancer, but it is already high in women with only a family history of breast cancer. Thus, being aware of the indication for a breast MRI screening exam might help to optimize the recall policy.
"Structured double reading with arbitration as is employed in mammography screening might help," Vreemann and Mann wrote in their email. "Finally, further development of AI [artificial intelligence] tools on larger datasets might bring them to the level required for clinical implementation, thus singling out relevant lesions without providing too much false-positive findings, first in order to prevent overlook errors, but hopefully also as a decision aid to reduce interpretation errors."
Regarding future research, the authors plan to focus on the consequences of missing breast cancer in prior scans -- for instance, the effects on cancer size and tumor stage. They will also study what to look for to prevent errors and the role AI can play in detection.