A multinational team has shown that the earlier and more frequently a woman starts screening for breast cancer, the higher her lifetime risk of radiation-induced cancer. But the total effective risk falls as women get older, particularly when they hit 70 years old, according to a study published in the European Journal of Radiology.
The researchers quantified radiation risk from screening mammograms by looking at the number of radiation-induced cancer cases per million (effective risk). They also simulated screening mammography through the use of breast phantoms. The research team, hailing from Iraq, the U.K., Sweden, and the Netherlands, found the most important parameters that contribute to effective risk of radiation-induced cancer from breast cancer screening are age and the number of screens.
"Graphical representation of risk could be an easy way to represent risk in a fashion that might be helpful to clients and clinicians," wrote lead author Raed M.K. M.Ali, PhD, a medical physicist at the University of Kufa in Iraq (EJR, November 2017, Vol. 96, pp. 98-103).
Quantifying risk
A person's radiation risk is determined by ionizing radiation that deposits energy in tissues. Factors that determine the risk include radiation dose, radiation source, exposure length, which organs are exposed, and the individual's sensitivity. Radiation risk falls into two categories: deterministic and stochastic. Deterministic health effects follow high radiation doses and result in direct and predictable tissue damage; stochastic effects follow low radiation doses and may result in cancer development.
The radiation risk from breast cancer screening is considered to be low, but the risks still exist.
"The health profession needs to understand the radiation risks to the woman from mammography imaging, in order to justify serial imaging at any frequency level," the study authors wrote.
Researchers often express radiation risk in terms of dose to the breast (mean glandular dose [MGD], which can be a difficult concept to understand by some imaging staff and patients alike. Is there an easier way to demonstrate radiation risk? That's precisely what the researchers of the current study sought to do.
To calculate effective risk, the researcher acquired organ dose data for all four mammography projections (craniocaudal and mediolateral oblique) along with lifetime attributable risk (LAR) factors for all ages that screening takes place ranging from 25, the earliest probable age of screening for high-risk patients in the U.S., to 75, the latest age of screening end worldwide. The researchers attached two breast phantoms to an adult dosimetry phantom and exposed the phantoms to 16 full-field digital mammography (FFDM) systems.
They calculated MGD; all other organ doses were measured directly using thermoluminescent dosimeters (TLD). LAR factors were calculated for a range of ages using a linear extrapolation method. Dose and LAR data were analyzed to generate scenarios to calculate total effective lifetime risk values.
The researchers proposed 274 different screening scenarios of different commencement/end ages (25 to 75 years old) and screening frequencies. For each proposed lifetime interval, such as 25 to 75 years old, 30 to 75 years old, and 30 to 70 years old, they scheduled three different screening categories with regard to screening frequency (annual, biennial, or triennial). The researchers generated a mathematical regression model and relationship establishment between total effective risk and number of screens and commencement/end ages using lifetime risk data.
The total MGD, for both craniocaudal and mediolateral oblique views, for the 16 FFDM machines was 2.019 mGy (1.871 to 2.166) mGy. The minimum recorded MGD was 1.678 mGy, while the maximum MGD was 2.806 mGy.
For organs other than the examined breast, for some organs the radiation dose was 0. However, some organs received radiation dose ranging from less than 1 µGy to more than 25 µGy. The contralateral breast tissue received the second highest radiation dose after the examined breast: 28.75 µGy. Sternum bone marrow radiation dose was the highest bone marrow dose and the third highest organ dose after the examined and contralateral breasts at 19.07 µGy.
In terms of total effective risk in general, the key findings are summarized in the line graph below.
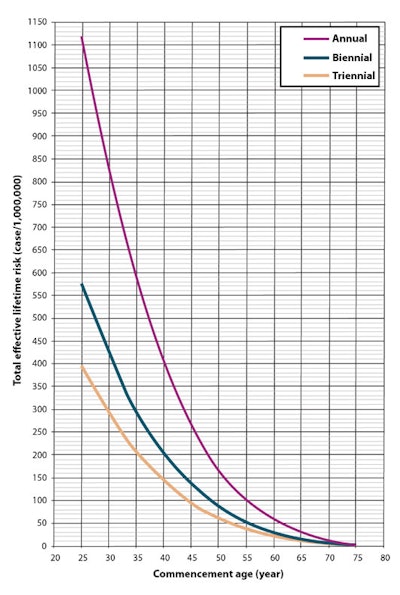
"This means that the total effective risk increases with early screening commencement age and a greater number of screens," the authors wrote. "However, a weak correlation is seen between the total effective risk and the end age of screening. This suggests that for any screening program the total effective risk decreases with increased end age of screening."
Future studies
The researchers did not take into account genetic factors in their statistical model, namely the BRCA1 and BRCA2 genes, and these factors are the subject of future research.
"Adding these factors to our statistical model as a covariate may lead to a refinement of our model and ultimately to a more accurate estimation of the true impact of mammography screening on the total effective risk," the authors wrote.
Also, the incorporation of breast density, breast size, genetic factors, and different screening procedures into the model would enable the estimation of risk to be personalized for individual screening clients, which the researchers also plan to study.