Assessing the response of tumors to chemoradiotherapy as rapidly as possible is critical for patient management. Traditionally, single static PET images have been used to make this assessment. For patients with head and neck cancer, pharmacokinetic modeling of F-18 fluoromisonidazole (FMISO) dynamic PET can provide a more detailed characterization of the tumor microenvironment and a more accurate assessment of response.
The increasing use of conformal technology to deliver precision high-dose radiation to a tumor target while minimizing dose to adjacent healthy tissue has improved the effectiveness of radiotherapy; as has radiobiology, which defines the mechanisms of radiation sensitivity and resistance in cancerous lesions. Better understanding of biologic processes and tumor response may facilitate adaptive radiotherapy, implemented early in the treatment.
Tumor hypoxia, the depletion of adequate oxygen supply at the tissue level, occurs as tumors grow and the growth exceeds their ability to generate the vascular support system. A reduction or absence of hypoxia in patients undergoing treatment may suggest the tumor is reoxygenating and becoming less radioresistant, and that remaining radiotherapy treatments could potentially be adjusted to a lower radiation dose.
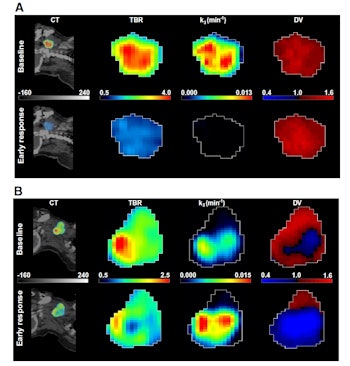
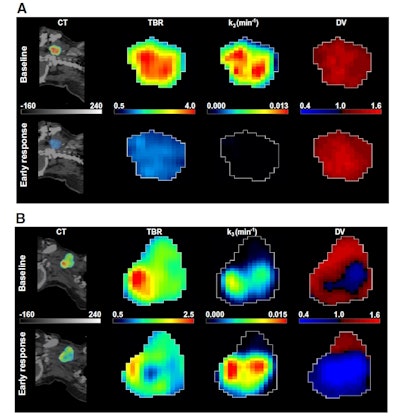
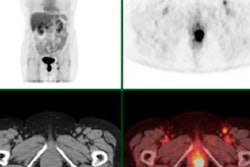
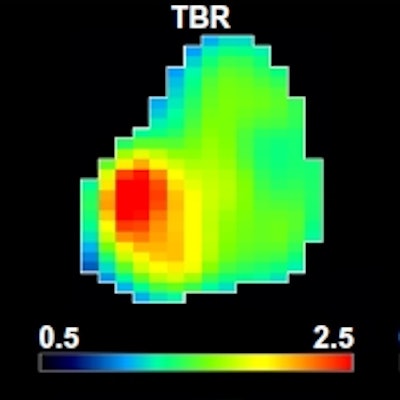
A: Patient No. 1, from left to right: sagittal view of the late 10-min FMISO PET/CT scan; tumor-to-blood ratio (TBR) map; k3 map representing hypoxia-mediated entrapment of FMISO; and FMISO distribution volume (DV), representing overall concentration of unbound FMISO relative to blood. For this patient both TBR and k3 decreased after one cycle of chemoradiotherapy, while DV did not change substantially. B: Corresponding images for Patient No. 2. For this patient, TBR decreased, while k3 increased. Decreased DV further contributed to underestimation of TBR. All images courtesy of Springer-Verlag, originally published in Milan Grkovski et al. European Journal of Nuclear Medicine and Molecular Imaging doi: 10.1007/s00259-017-3720-6.
Researchers at Memorial Sloan Kettering Cancer Center in New York have evaluated tumor microenvironment characteristics in patients with squamous cell carcinoma of the head and neck, using imaging data from dynamic FMISO PET. They assessed tumor hypoxia, tumor perfusion, and FMISO distribution volume, to evaluate the capabilities of pharmacokinetic modeling to assess early response after patients had completed one chemotherapy cycle and received five to 10 fractions of their radiation treatment (European Journal of Nuclear Medicine and Molecular Imaging, 24 May 2017).
Interpreting PET data
The study included 72 patients who had a FDG PET/CT scan at baseline, followed by a baseline FMISO dynamic PET/CT scan and an additional FMISO dynamic PET/CT scan after initial treatment. The researchers identified 54 hypoxia-positive primary tumors and 81 metastatic lymph nodes, defined by increasing or stable FMISO uptake in the known disease location during the time period of the three scans.
An experienced nuclear medicine physician analyzed these lesions visually to derive tumor-to-muscle ratios (TMR), with TMR greater than 1.2 defining hypoxia. The images also were analyzed by voxel-wise pharmacokinetic modeling using an irreversible one-plasma two-tissue compartment model to calculate surrogate biomarkers of tumor hypoxia (the kinetic rate constant k3 and tumor-to-blood-ratio), perfusion and FMISO distribution volume.
The authors reported that 69 out of 72 patients were hypoxia-positive on baseline scans, having at least one lesion with TMR greater than 1.2. Hypoxia resolution was observed on the post-treatment scans in 20 of these 69 patients. For 13 patients, TMR was below 1.2 on early response scans; however, pharmacokinetic modeling indicated the presence of hypoxic regions. Hypothetically, if a clinical trial led to a de-escalation of therapy for patients with an indicated absence of hypoxia, patient management would have been affected in these 13 cases.
FMISO's future
In the future, the researchers intend to perform a survival analysis of the patient group to evaluate the clinical relevance of the disagreement between static and dynamic PET data. Survival analysis may help answer the question as to whether regions that contain severely hypoxic cells can be assumed to contain clinically relevant hypoxia, a predictor of poor survival. For a subset of patients who also received dynamic contrast-enhanced and diffusion weighted imaging MRI scans, the researchers are currently working on correlating PET- and MRI-derived metrics to better understand the discrepancy between total FMISO uptake and k3.
The authors stated that "establishing a noninvasive and quantitative measurement of the tumor hypoxic burden is an important first step in testing the clinical relevance of hypoxia and its utility as a tumor response biomarker." Lead author Milan Grkovski told medicalphysicsweb this would enable physicians to more efficiently stratify patients.
"Those with low levels of clinically relevant hypoxia could be candidates for dose de-escalation, whereas those with high focal areas of hypoxia might be candidates for intensity-modulated dose painting to putative radioresistant subregions," he explained. "Another possibility is identification of patients who might benefit from hypoxia-activated prodrugs."
How else might FMISO dynamic PET be clinically used? The authors suggest it might be appropriate for cancers exhibiting a wide range of distribution volumes (due to the presence of necrotic or adipose-like tissue, for example), and having relatively poor perfusion, lower vascular permeability, and longer FMISO equilibrium times. In such scenarios, total FMISO uptake is a function of several variables, whereas pharmacokinetic modeling would enable to extract a kinetic rate constant k3 that, in principle, more directly represents the degree of hypoxia.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.