An Italian CT lung cancer screening trial has found low-dose CT (LDCT) screening could reduce lung cancer and overall mortality, and it is the first European study to show results clearly in the same direction of other major lung cancer screening trials.
The Italian CT Lung Cancer Screening Trial (ITALUNG) research team, led by Dr. Eugenio Paci from the Cancer Research and Prevention Institute (ISPO) in Florence, randomized smokers or ex-smokers to receive LDCT screening for four years or for usual care.
"The ITALUNG study has confirmed that LDCT screening, in conjunction with improvement of treatment strategies in early-stage lung cancer cases and effective national policies for smoking cessation, is an important tool for the reduction of deaths from lung cancer," Paci and colleagues wrote (Thorax, 4 April 2017).
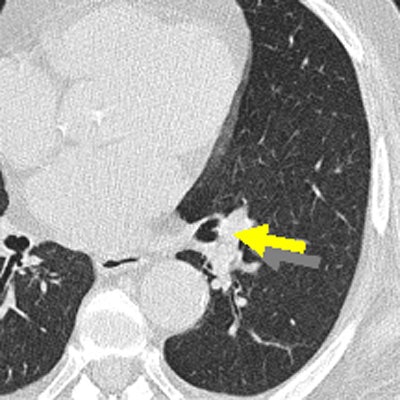
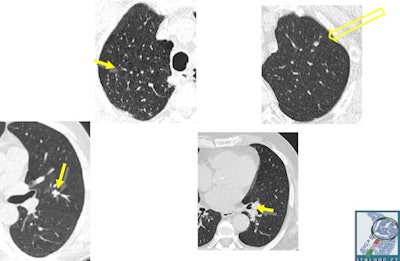 Initial aspects of malignant lesions in the ITALUNG study: squamous adenocarcinomas. Image courtesy of Drs. Mario Mascalchi, Fabio Falaschi, and Giulia Picozzi.
Initial aspects of malignant lesions in the ITALUNG study: squamous adenocarcinomas. Image courtesy of Drs. Mario Mascalchi, Fabio Falaschi, and Giulia Picozzi.Adding to the literature
Lung cancer is the leading cause of cancer deaths in men and the third leading cause in women in Italy, the authors noted. Screening for lung cancer with LDCT in the U.S. National Lung Screening Trial (NLST) reduced lung cancer mortality by 20%, and the guidelines for lung cancer screening were updated so that LDCT screening is currently recommended for high-risk patients in the U.S. Conversely, public health guidelines in Europe do not recommend screening for lung cancer, because the evidence of its benefits and harms has not been considered sufficient.
The ITALUNG lung cancer screening trial was launched in 2004 in Tuscany with the aim of contributing to the European evaluation of the efficacy of LDCT screening for reducing lung cancer-specific and overall mortality, as well as assessing the benefit-to-harm ratio.
Other lung cancer screening studies, such as DANTE, MILD, and DLST, have already published their first results. The largest lung cancer screening study, NELSON, is awaiting publication. ITALUNG is the first European study showing results in line with NLST, according to the authors.
Smokers or ex-smokers, who smoked at least 20 pack years in the last 10 years, from Florence, Pisa, and Pistoia from the ages of 55 to 69 were randomized to receive an annual invitation for LDCT screening for four years (study group) or usual care (control group). All participants were followed up for vital status and cause of death at the end of 2014 and lung cancer incidence at the end of 2013.
After the nine-year follow-up, the study group included 1,613 participants with 67 lung cancer cases. The control group included 1,593 people with 71 lung cancer cases. Also, the researchers observed a greater proportion of stage I lung cancers in the study group (36% versus 11%). They estimated nonsignificant reductions of 17% for overall mortality and 30% for lung-cancer specific mortality in the study group.
The researchers found no difference in mortality during the screening phase (the four years following randomization), whereas they saw a significant reduction in both lung cancer-specific (p = 0.01) and overall mortality (p = 0.045) after the screening period.
How ITALUNG compares
"Notably, the decrease in mortality observed in our study was larger than that reported in the NLST, in which decreases of 20% and 7% in lung cancer-specific and overall mortality, respectively, were observed at 6.5 years of median follow-up," the study authors wrote. This is most likely due to the longer follow-up and subjects enrolled in the control group were not invited to screening but received usual care, whereas in the NLST the control group underwent chest posteroanterior radiographs.
Further studies are necessary to confirm the ITALUNG results, but the comparison of the number of lung cancer cases diagnosed in the two groups does not suggest overdiagnosis after an adequate follow-up period, according to Paci and colleagues. The impression is supported by the results of the survival analysis, which show no difference if surgical treatment is taken into account.
The lung cancer incidence pattern in the ITALUNG trial confirmed the high sensitivity of low-dose CT screening and adds to the results of other LDCT trials. None of the previously published outcome studies, including the ITALUNG trial, has sufficient statistical power alone to detect a real benefit, the authors noted.
"A pooled analysis of all European trials including NELSON and the ongoing UKLS and LUSI randomized controlled trials is thus becoming a crucial step in assessing the expected benefit of LDCT screening in Europe," Paci and colleagues concluded.
And before lung cancer screening programs become commonplace in Europe, several critical issues still need to be addressed, such as optimization of recruitment and also definition of more efficient protocols for nodule management and the optimal screening interval, they added.
More new evidence
In an article published recently in the International Journal of Cancer (7 April 2017), further research on the ITALUNG trial enrolled asymptomatic high-risk subjects (n = 1,356) to examine biomarkers. The group, led by Francesca Maria Carozzi from the Regional Cancer Prevention Laboratory at ISPO, found multimodal screening could improve the screening efficiency at baseline. If biomarkers were used as a primary screening test, the LDCT burden might decrease by about 60%.
The researchers collected samples of blood and sputum, analyzing for plasma DNA quantification (cutoff 5 ng/mL), loss of heterozygosity, and microsatellite instability. The ITALUNG biomarker panel was considered positive if at least one of the two biomarkers included in the panel was positive.
Of the 18 baseline screen-detected lung cancer cases, 17 (94%) were positive. There were also 18 repeat screen-detected lung cancer cases, and 12 (66%) of them were positive at the baseline biomarker test.
In terms of future research, the investigators plan to combine multimodal screening for lung cancer (LDCT and biomarker) in already-stored data from other ongoing trials and in an observational study considering high-risk subjects. They also hope to cooperate with other European groups.