Publication of the results from joint French-Singaporean research based on hepatocellular carcinoma (HCC) therapy trials involving 800 patients promises to add fresh impetus to the debate over the optimal treatment for advanced nonresectable liver cancer.
Researchers in oncology at Paris Public Hospitals (Assistance Publique- Hôpitaux de Paris, AP-HP), the Asia-Pacific Hepatocellular Carcinoma Trials Group (AHCC), National Cancer Center Singapore (NCCS), and the Singapore Clinical Research Institute (SCRI) have confirmed their future partnership for a prospective meta-analysis that will combine the upcoming results from two large, randomized, controlled trials aiming to compare resin microspheres containing yttrium-90* (Y-90) and sorafenib.
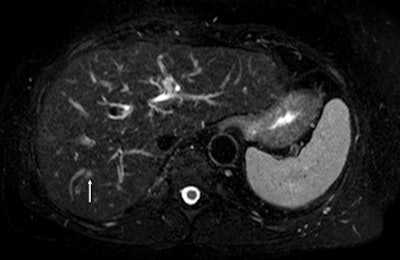 Fat-suppressed T2-weighted fast spin-echo MR image obtained in 61-year-old man with liver metastases from colorectal cancer. Only one metastasis in the right liver lobe is seen on this slice (arrow). Moreover, this lesion is barely seen because it is located next to a vessel. Images courtesy of Dr. Valérie Vilgrain, PhD.
Fat-suppressed T2-weighted fast spin-echo MR image obtained in 61-year-old man with liver metastases from colorectal cancer. Only one metastasis in the right liver lobe is seen on this slice (arrow). Moreover, this lesion is barely seen because it is located next to a vessel. Images courtesy of Dr. Valérie Vilgrain, PhD.
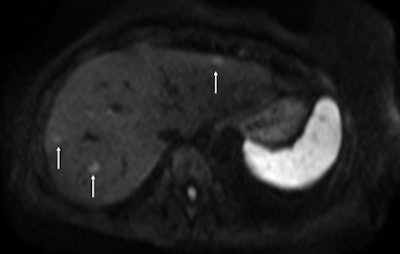 On the diffusion-weighted image, the same lesion is seen as well as two additional metastases: one in the right liver and one in the left liver. All metastases are strongly hyperintense compared to the background liver at b = 600 sec/mm2, indicating restricted diffusion. All of these lesions were surgically confirmed as metastases.
On the diffusion-weighted image, the same lesion is seen as well as two additional metastases: one in the right liver and one in the left liver. All metastases are strongly hyperintense compared to the background liver at b = 600 sec/mm2, indicating restricted diffusion. All of these lesions were surgically confirmed as metastases.The studies, for which recruitment has finished, are based on 800 patients suffering with primary advanced cancer of the liver. The French study, SARAH, is being promoted by AP-HP and conducted across more than 25 specialist centers in France. The trial undertaken in Singapore, SIRveNIB (AHCC protocol 06), is being conducted in 27 regional centers and uses similar research models to examine the efficacy, the safety profile, and the quality of life of selective internal radiation therapy (SIRT) using Y-90 resin microspheres targeting the liver, compared with sorafenib, currently the standard treatment for advanced HCC, via systemic chemotherapy. Both trials are financially supported by Sirtex.
Patients with HCC recruited to SARAH and SIRveNIB were not eligible for curative treatment, such as surgical resection, ablation, or liver transplant, and furthermore, chemoembolization was either ineffective or inappropriate, according to a statement released by the partners.
Results from the prospective meta-analysis will be available in 2017, and ahead of this, details of the methodological and statistical approach of the meta-analysis will be published in a peer-reviewed journal.
"The research into the most effective and best tolerated treatment for HCC is crucial because of the low number of proven options currently in existence," noted Dr. Pierce Chow, principal investigator of SIRveNIB and senior consultant surgeon at the National Cancer Center of Singapore and Singapore General Hospital, in the statement.
"Our study is based on more than 360 patients from 27 specialist centers across 10 countries in the Asia-Pacific region. Even if our data is published independently, to be able to combine them within a prospective meta-analysis with the results of the French study, SARAH, constitutes a significant scientific achievement," he continued. "Involving a larger patient population will generate an increased amount of data available for diverse planned statistical analysis, notably in overall survival."
 Dr. Valérie Vilgrain, PhD. Image courtesy of the European Society of Radiology.
Dr. Valérie Vilgrain, PhD. Image courtesy of the European Society of Radiology.Doctors treating HCC will enjoy a greater degree of certainty with regard to the applicability of the results to the treatment of this often fatal cancer, Chow added.
Dr. Valérie Vilgrain, PhD, principal investigator of the SARAH study and professor and head of radiology at Beaujon Hospital, AP-HP reinforced the message.
"Even if results from SARAH are also published separately, we think that combining our results with those from SIRveNIB in a prospective meta-analysis could prove highly interesting," she stated. "In France and in nearly all of Europe, HCC is diagnosed in patients with liver cirrhosis essentially due to the hepatitis C virus and alcohol abuse, while in the majority of cases in Asia it is caused by the hepatitis B virus. Therefore our prospective meta-analysis will provide data about the safety and efficacy for patients from a large part of the HCC-associated etiological spectrum, which will potentially increase the clinical applicability of the results."