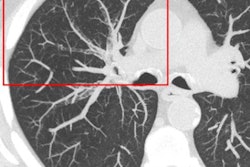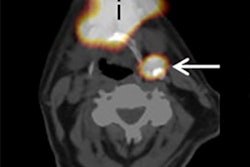
Molecular imaging is essential for research of human disease models in laboratory animals, enabling noninvasive visualization of minute structures at the sub-organ and/or sub-tumor level. Its use has been constrained, however, by the challenge of producing images with high enough spatial resolution. The development of multipinhole collimators represented a breakthrough, enabling radionuclide imaging with quarter-millimeter resolution.
Researchers from the Netherlands have reported on the capabilities of a new-generation SPECT/CT system equipped with an ultrahigh-resolution collimator to perform in vivo molecular imaging (Molecular Imaging, 27 November 2014).
MILabs (Utrecht, the Netherlands), a commercial spinoff from the University Medical Center Utrecht, has been at the forefront in the development of ultrahigh-resolution molecular imaging systems since 2006. Its U-SPECT+/CT system contains three large stationary gamma-ray detectors (595 x 472 mm) with 9.5 mm thick NaI(TI) crystals, with exchangeable cylindrical pinhole collimators placed in the center of the system.
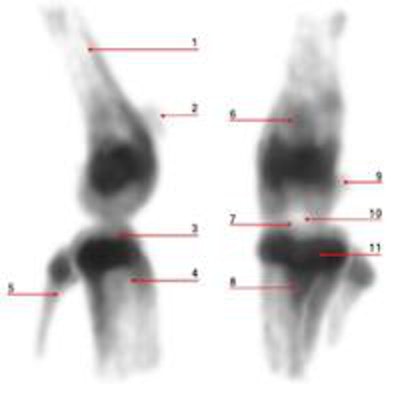
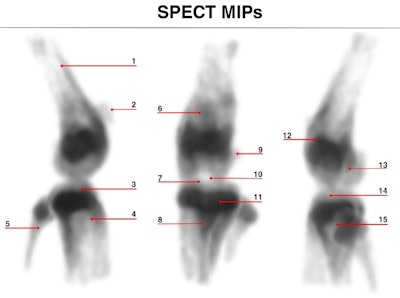 Maximum intensity projections of focused mouse knee joint SPECT (top) and corresponding micro-CT scan (bottom). The SPECT images provide an unprecedented level of uptake details in a mouse knee such as in structures like corpus femoris (labeled 1), patella (2), tibia, condylus medialis (3), corpus tibiae (4), corpus fibulae (5), basis patellae (6), linea epiphysialis (7), tuberositas tibiae (8), sesamoid bone (9), tuberculum intercondylare laterale (10), growth plate (11), epicondylus lateralis (12), fossa intercondylaris (13), tibia, condylus lateralis (14) and apex capitis fibulae (15). The CT scan below the SPECT images shows the corresponding structural details of the same mouse knee.
Maximum intensity projections of focused mouse knee joint SPECT (top) and corresponding micro-CT scan (bottom). The SPECT images provide an unprecedented level of uptake details in a mouse knee such as in structures like corpus femoris (labeled 1), patella (2), tibia, condylus medialis (3), corpus tibiae (4), corpus fibulae (5), basis patellae (6), linea epiphysialis (7), tuberositas tibiae (8), sesamoid bone (9), tuberculum intercondylare laterale (10), growth plate (11), epicondylus lateralis (12), fossa intercondylaris (13), tibia, condylus lateralis (14) and apex capitis fibulae (15). The CT scan below the SPECT images shows the corresponding structural details of the same mouse knee.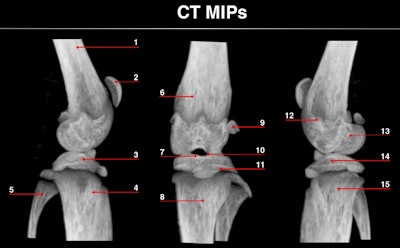
For the study, the system was equipped with mouse SPECT collimators with five rings of 0.25 mm diameter circular pinholes. All of the pinholes are focused on a common region that can be reconstructed without any bed movement. The only moving part is an automated robotic arm connected to an animal bed that shifts the animal through the scanner during SPECT data acquisition, in either a spiral trajectory or a multiplanar stepwise mode.
This system is more advanced than its predecessor with regard to the mechanical accuracy of the robotic stage and the collimators, and optimized data sampling with spiral bed trajectories for data collection. MILabs also improved the image reconstruction capabilities using a more robust fitting procedure of the geometry of the system. Detailed attenuation correction (based on optical or x-ray CT images) was added to provide absolute quantification.
Lead author Oleksandra Ivashchenko of the Technical University of Delft (TU Delft) and co-authors measured the sensitivity of the U-SPECT+/CT with the 0.25 mm pinhole collimator by imaging a technetium-99m (Tc-99m) point source. Results showed the sensitivity at the center of the collimator was 380 cps/MBq. They determined the reconstructed spatial resolution by imaging a hot-rod resolution phantom with six pie-shaped sectors containing rods with diameters of 0.22, 0.25, 0.3, 0.35, 0.4, and 0.5 mm. With the 0.25 mm pinhole collimator, the 0.25 mm rod was clearly visualized.
The researchers also performed bone imaging on three mice: total-body scans and focused scanning of the knee joint and lumbar spine, each taking 90 minutes. Bone turnover in the entire skeleton of the mouse could be visualized in great detail in the total body scans. Ribs and individual sternebra bones were fully visible, as well as the scapulae and clavicles. The ilium, pubis, and scarum in the pelvis area of the mouse showed many anatomic structures.
Reconstructed images of the mouse knee joint showed detailed joint substructures. "The visibility of details was at such a level that the radiotracer uptake in joint substructures, such as the linea epiphysialis and intercondylar tuberculum of the tibia, could be distinguished," Ivashchenko said. "This is essential for multiple areas of bone research. In addition, time-activity curves of the lumbar spine illustrated that tracer dynamics in tiny tissue amounts could be measured. It allows monitoring biological processes in tinier parts of organs in vivo than ever before."
She added: "These unique properties make small-animal SPECT more attractive for replacing optical imaging and PET, as well as several ex vivo methods. Not only may the need for additional anatomical imaging become less important, but this may reduce labor and the number of laboratory animals that need to be sacrificed compared to postmortem tissue distribution studies."
In initial scans, Ivashchenko and co-workers used an average of 8.9 mCi Tc-99m-MDP resulting in 44 cGy total body dose for a 30 g mouse, which is 20 times below the lethal dose 50/30. Next, the researchers showed that imaging with a 10 times lower dose still resulted in almost equally highly detailed knee images. For imaging with dramatically lower doses (for example, about 7 µCi), the teams at TU Delft and MILabs have jointly developed other submillimeter collimators suitable for cases when, for example, a very low amount of tracer is available.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.