Elekta has announced its plans for the commercial release of Atlantic -- a high-field MRI-guided radiation therapy system. Developed by a research consortium established by Elekta, Philips Healthcare, and University Medical Center (UMC) Utrecht in the Netherlands, the new treatment system will be launched in 2017, with the first units delivered a year later.
"Based on where we are in the project, we believe that Atlantic will receive the CE Mark and FDA 510(k) clearance during 2017, and we will start shipping the first commercial systems during 2018," said Elekta's President and CEO Niklas Savander. Speaking at an Elekta R&D update held last week at UMC Utrecht, Savander predicted that around 75 systems will be ordered and delivered during the ramp-up phase into 2019.
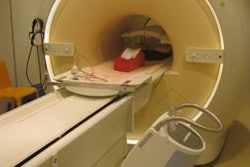
 Bas Raaymakers (left), professor of experimental clinical physics at UMC Utrecht, in front of Elekta's Atlantic system with Robert Spaninks, Elekta service engineer. Image credit: PRNewsFoto/Elekta
Bas Raaymakers (left), professor of experimental clinical physics at UMC Utrecht, in front of Elekta's Atlantic system with Robert Spaninks, Elekta service engineer. Image credit: PRNewsFoto/Elekta"Speaking with the research consortium, and the huge amount of hospital CEOs and researchers who have contacted Elekta about this, we do think this will have the potential to go mainstream and really become a significant new part of radiation therapy," he said. "It is that interest from leading hospitals around the world that gives us that confidence that we are on the right track."
Atlantic will be priced at around four times that of the Versa HD, Elekta's top-end radiotherapy system that offers treatments including stereotactic radiosurgery and volumetric-modulated arc therapy using the Agility 160-leaf multileaf collimator (MLC) and cone-beam CT guidance. "With Versa HD, we have the technology platform to deliver a highly precise radiation beam," said Dee Mathieson, senior vice president of Elekta Oncology. "The challenge we now face is to place that precise beam more accurately. And we believe the way to do that is using high-field MRI."
Mathieson explained the high-quality images provided by Atlantic will enable more accurate placement of dose and give users the confidence to shrink treatment margins. The high-field MRI will potentially also enable functional imaging to provide information about changes in the patient at the biological level. "We will be able to deliver a gentler treatment, with fewer side effects, potentially using fewer fractions and with the possibility of treating more indications," she said.
Ongoing development
Kevin Brown, Elekta's global vice president of scientific research, described the evolution of the MR-guided radiotherapy system from an experimental device into a commercial system. He explained that the team initially built an experimental device simply to prove that such an integrated system could actually work.
And it did. The researchers demonstrated 1.5-tesla MR image quality from the magnet, even though it had been modified, and showed that image quality was unaffected by the presence of the radiation beam or the linac gantry. They also demonstrated full operation of the linac in the presence of the MR system, albeit with a small impact of the gantry speed on the image quality.
The commercial-grade system, the first of which is now being installed at UMC Utrecht, was then built to demonstrate that, "not only does the concept work, but that we have the design right, that we can manufacture and service it, and it is reliable -- all the things you'd expect of a modern medical system." Repeating the proof-of-concept tests on this system revealed that it met, or exceeded, all specifications.
"The only difference was that, due to improvements in the magnet design, the coupling between the linac gantry and the MR imaging has actually reduced," Brown noted. "Now we're fully within specifications even when the gantry is rotating."
The Atlantic can also acquire high-speed cine MR movies and process these in real time. "We have acquired and processed MR images to identify the target position in under 200 msec," Brown said. He suggested this facility could enable dynamic tumor tracking, citing tests on the Versa HD (using the same Agility MLC as on the Atlantic) that demonstrated high-accuracy, low-latency tracking of a moving target.
"The Agility MLC, and hence Atlantic, is ideally suited to dynamic tracking because of its speed; we measured the latency as 0.1 sec, that's effectively instantaneous," Brown explained. "The dose distributions showed that it's like treating a moving target with the same result as if that target was stationary."
The Atlantic consortium currently includes seven partners -- two in the U.S., U.K., and the Netherlands, and one in Canada -- each of whom will receive a commercial-grade system. Once installed, the systems will be used for both technical and clinical research, and ultimately to perform clinical trials. Brown noted the Atlantic is comparable in size to the Versa HD, and all of the partners could fit the machine in an existing treatment vault.
"The consortium aims to demonstrate that this technology will improve outcomes for existing radiotherapy treatments," Brown said. "We are also exploring new treatment techniques and new indications that will be enabled by this technology."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.