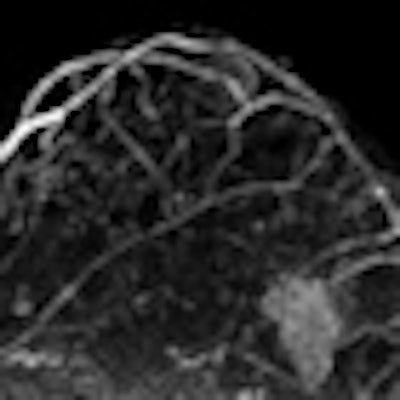
In a head-to-head comparison, gadobenate dimeglumine contrast offered significantly improved diagnostic performance in breast MR compared to gadopentetate dimeglumine, according to a study published online December 16 in Radiology.
The team, led by Laura Martincich, MD, from the Institute for Cancer Research and Treatment in Turin, Italy, also concluded that MRI is "an accurate tool for screening women at high risk of breast cancer." In addition, the results "highlight its value for staging breast cancer, determining the most appropriate treatment, and following up patients after breast cancer treatment," they wrote.
The study, entitled Phase III Multicenter Double-Blind, Randomized, Crossover Study to Compare MultiHance With Magnevist in Contrast-enhanced Magnetic Resonance Imaging (MRI) of the Breast (DETECT trial), compared the gadobenate dimeglumine contrast agent from Bracco of Milan with Magnevist, the gadopentetate dimeglumine contrast agent from Bayer HealthCare Pharmaceuticals of Wayne, NJ. Bracco sponsored the study.
A total of 162 women were enrolled from 17 healthcare facilities in Europe and China between July 2007 and May 2009. Individuals were included in the study if they were found to have a suspicious abnormality on their mammograms or ultrasound exams. They also were highly likely to undergo biopsy or surgery, according to the authors.
The participants had a mean age of 52.8 years, ranging from 24 to 87. The researchers randomized the enrollees into two groups: One group with 82 subjects received gadobenate dimeglumine for the first examination and gadopentetate dimeglumine for the second exam. The second randomized group of 80 enrollees received both contrast agents in reverse order.
MRI protocol
All procedures were performed on 1.5-tesla MRI systems (Sonata, Avanto, or Symphony, Siemens Healthcare, Erlangen, Germany; Achieva or Intera, Philips Healthcare, Andover, MA; Signa Excite or Genesis Signa, GE Healthcare, Chalfont St. Giles, U.K.).
The contrast agents were administered intravenously using a power injector in 158 women (98%), at a rate of 2 mL/sec in 139 women (86%), 1.8 mL/sec in two women, and 1.5 mL/sec in 17 women (0.1%). The agents were administered as a manual bolus in four cases (3%) at a rate of 1-2 mL/sec for approximately 10 seconds to ensure the same rate was used for both examinations in each patient.
All images were evaluated independently by three radiologists -- including lead study author Martincich -- who were not affiliated with the 17 participating centers. Readers also were blinded to the contrast agent used in each examination, to patients' clinical and radiologic information, and to the results of all interpretations by onsite investigators.
In addition, all women were monitored for any adverse events from the contrast agents beginning at the time of consent until 24 hours after administration of the first contrast agent, and again from 24 hours before until 24 hours after administration of the second contrast agent.
Eleven women were excluded from the final results after not proceeding in the study following the first exam. Seven women discontinued the study after their MRI exam with gadobenate dimeglumine, while four women halted participation after their MRI scan with gadopentetate dimeglumine. One additional patient was excluded from evaluation because of contrast agent extravasation during the first examination.
Contrast administration
In the final analysis, 157 women received gadobenate dimeglumine and 155 women were given gadopentetate dimeglumine. A total of 150 evaluable subjects received both contrast agents.
A diagnosis was available for 216 lesions in 136 (91%) of the 150 women available for assessment. A total of 144 malignant and 52 benign lesions were confirmed with histopathologic examination in 132 patients, and 20 benign lesions were confirmed with follow-up in 10 patients. (Five subjects had both histologically confirmed malignant and benign lesions; six women had both malignant and benign lesions confirmed with follow-up.)
The image analysis by Martincich and colleagues found "significantly superior cancer detection with gadobenate dimeglumine" among all three readers. Use of gadobenate dimeglumine identified 17 noninvasive cancers, compared to the 11 identified with gadopentetate dimeglumine.
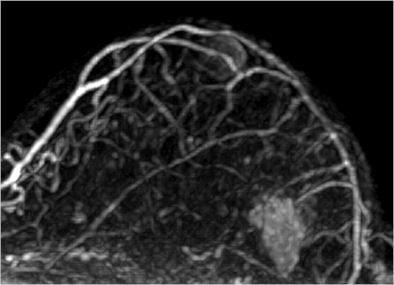 |
| Image is of a 50-year-old woman with a family history of breast cancer. The patient received MRI following inconclusive mammography and ultrasound findings. Evaluation of images in matched pairs confirmed unanimous reader preference for gadobenate dimeglumine for lesion conspicuity, border delineation, and overall diagnostic performance. Image courtesy of Radiology. |
In addition, a total of 1,530 breast regions were assessed by the readers, with 1,450 regions evaluated for both contrast agents. All three readers generated significantly superior sensitivity, specificity, and accuracy with gadobenate dimeglumine for the detection of breast cancer. Similarly, "highly significant superiority" was noted by the authors for positive predictive value and negative predictive value.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: Radiology | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Negative predictive values were greater than 97% and significantly higher for gadobenate dimeglumine compared to gadopentetate dimeglumine, according to the researchers. Positive predictive values also were significantly higher for gadobenate dimeglumine, they wrote.
Adverse reactions
Among the subjects, several women experienced adverse reactions from the contrast agents. Eleven incidents among seven women were reported with gadopentetate dimeglumine, with four cases of nausea, two of dizziness, two of dysgeusia, and one each of vomiting, vertigo, and headache. There were eight reactions to gadobenate dimeglumine among six patients, with two cases of dizziness, two of vertigo, and one each of dysgeusia, decreased blood pressure, increased heart rate, and an abnormal electrocardiogram.
The researchers described the adverse reactions as nonserious and mild, and they resolved spontaneously within 24 hours. The group found no differences in vital sign measurements or electrocardiograms.
Regarding study limitations, the authors noted that there was no comparison with mammography or ultrasound, and they did not provide an analysis according to lesion type.
By Wayne Forrest
AuntMinnie.com staff writer
December 22, 2010
Related Reading
New FDA policy has changes for all gadolinium MRI agents, September 16, 2010
MGH study: Adverse reactions to gadolinium contrast are rare, January 22, 2010
Italian researchers cut gadolinium dose -- as well as NSF risk, January 21, 2010
FDA panel: NSF incidence falls with gadolinium restrictions, December 9, 2009
Copyright © 2010 AuntMinnie.com