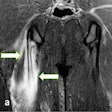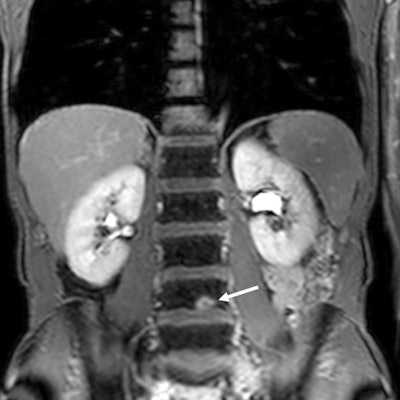
Fresh evidence shows that whole-body MRI may be quicker and cheaper than standard imaging for detecting the spread of colorectal and non-small cell lung cancers, while being just as sensitive. The findings from two prospective trials involving nearly 500 patients across 16 U.K. hospitals were published on 9 May in Lancet Gastroenterology & Hepatology and Lancet Respiratory Medicine.
 Prof. Stuart Taylor now is planning trials of staging whole-body MRI in other primary cancer sites, notably the breast.
Prof. Stuart Taylor now is planning trials of staging whole-body MRI in other primary cancer sites, notably the breast."Our results, obtained in a real-world NHS [National Health Service] setting, suggest that whole-body MRI could be more suitable for routine clinical practice than the multiple imaging techniques recommended under current guidelines," said lead author Prof. Stuart Taylor, professor of medical imaging at University College London. "While demands on NHS MRI scanners are currently high, MRI can image the whole body in one hour or less. Adopting whole-body MRI more widely could save rather than increase costs, as well as reducing the time before a patient's treatment can begin."
In patients with newly diagnosed colorectal and non-small cell lung cancer, Taylor and colleagues found that whole-body MRI scans reduced the average time to determine the size of tumors and how much they had spread by five days for colorectal cancer patients and six days for lung cancer patients. The treatments decided upon were similar because results from MRI were as accurate as those from standard investigations, but the costs per patient were reduced by nearly a quarter in the case of colorectal cancer and were almost halved for lung cancer.
Study details
In research funded by the U.K. National Institute for Health Research, the authors screened 1,020 patients between March 2013 and August 2016 and recruited 370, of whom 299 completed the colon trial. Pathway sensitivity was 67% (95% confidence interval [CI]: 56% to 78%) for whole-body MRI and 63% (CI: 51% to 74%) for standard pathways, a difference in sensitivity of 4% (CI: -5% to 13%, p = 0.51). No adverse events related to imaging were reported. Specificity did not differ between whole-body MRI (95%, CI: 92% to 97%) and standard pathways (93%, CI: 90% to 96%, p = 0.48).
For the lung trial, the so-called Streamline L trial, 976 patients were screened for eligibility; 353 patients were recruited, of whom 187 completed the trial. Pathway sensitivity was 50% (95% CI: 37% to 63%) for whole-body MRI and 54% (CI: 41% to 67%) for standard pathways, a difference of 4% (CI: -7% to 15%, p = 0.73). No adverse events related to imaging were reported. Specificity did not differ between whole-body MRI (93%, CI: 88% to 96%) and standard pathways (95%, CI: 91% to 98%, p = 0.45)
| Whole-body MRI for detecting cancer metastasis | ||||
| Streamline C (colorectal) | Streamline L (lung) | |||
| Standard patient workup | Whole body-MRI | Standard patient workup | Whole body-MRI | |
| Sensitivity* | 63% | 67% | 54% | 50% |
| Specificity | 93% | 95% | 95% | 93% |
| Time to complete staging | 13 days | 8 days | 19 days | 13 days |
| Cost | 285 pounds (326 euros) | 216 pounds (247 euros) | 620 pounds (709 euros) | 317 pounds (362 euros) |
Taylor and colleagues also followed up patients after 12 months to better evaluate the accuracy of whole-body MRI compared with standard tests -- e.g., to find out whether one approach was more sensitive than the other in detecting the spread of the primary tumor to other parts of the body. Based on the data, they were able to retrospectively evaluate what the optimal treatment decision should have been.
In the colorectal cancer trial, agreement with the final multidisciplinary panel treatment decision based on standard investigations and whole-body MRI were similar and high (95% and 96%, respectively), as were results for the lung cancer trial (99% for standard investigations and 98% for whole-body MRI).
Expensive technology?
The team was not really surprised about any of the major results but found it interesting that the costs of the whole-body MRI pathway were less than those of the standard pathway using English NHS cost tariffs, according to Taylor.
"Whole-body MRI is viewed as an expensive technology. The overall sensitivity of both staging pathways was at the lower limits of that reported in the literature but this reflects the pragmatic trial design and 'real world setting,' " he told AuntMinnieEurope.com.
Taylor said he would like to have assessed the impact of dedicated workshop-based training of reporting radiologists. "However, a strength of the study design is that we replicated the likely training radiologists would undergo should whole-body MRI be more widely disseminated," he pointed out.
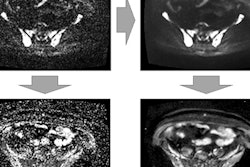
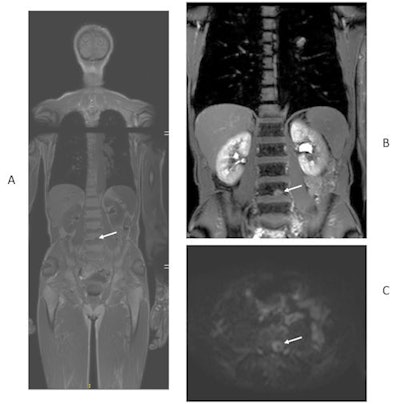 49-year-old man with newly diagnosed lung cancer recruited to Streamline L trial. Whole-body MRI revealed a solitary L4 bone metastasis that was occult on standard imaging, including CT and PET/CT. A = coronal T1-weighted image. B = contrast-enhanced T1-weighted image. C = axial diffusion-weighted image. Figure courtesy of Prof. Stuart Taylor.
49-year-old man with newly diagnosed lung cancer recruited to Streamline L trial. Whole-body MRI revealed a solitary L4 bone metastasis that was occult on standard imaging, including CT and PET/CT. A = coronal T1-weighted image. B = contrast-enhanced T1-weighted image. C = axial diffusion-weighted image. Figure courtesy of Prof. Stuart Taylor.He thinks the results are equally applicable in the rest of Europe because cancer-staging protocols are largely uniform across the continent. Scan costs are likely to vary between different countries, but Taylor and his colleagues have provided a detailed breakdown of the actual scans performed so other researchers can easily do their own cost analysis.
Writing in a linked comment, Prof. Andreas Schreyer from Brandenburg Medical School in Germany stated the following: "MRI has faced considerable backlash within the medical community due to relatively high costs and the problems involved in finding a timely slot for imaging because of the high demand for this method. This is why it is particularly important to think outside the box and look out for new medical pathways and paradigms and not to be driven by prejudices."
It could be more efficient to adapt the known therapeutic concept of hitting hard and early to diagnostic imaging to improve medical outcomes and economic performance, he added.
In terms of the limitations of the study, eight of the 16 hospitals in the colorectal cancer trial and 11 of the 16 hospitals in the lung cancer trial did not have the infrastructure to perform whole-body MRI. Furthermore, the authors noted that their findings are specific to colorectal and non-small cell lung cancer and might not be relevant to tumors arising in other parts of the body, and waiting times might not be representative of other U.K. hospitals or of hospitals in other countries.
Another limitation of the lung cancer trial is that sensitivity in detecting the spread of cancers -- including the development of secondary tumors and the spread to lymph nodes -- was low using both current standard imaging techniques and whole-body MRI. More research is needed to improve the performance of noninvasive imaging, the authors believe.
Also, more research is needed to determine how the results affect outcomes for patients. Appropriate treatment cannot be decided upon until the size of a tumor and the extent to which it has spread to nearby lymph nodes and other parts of the body has been determined, they noted. Standard NHS pathways often involve different imaging techniques -- such as CT, PET/CT, or focused MRI scans -- which vary in accuracy in different organs, so several appointments and follow-up examinations may be necessary.
Future goals and plans
Looking ahead, Taylor hopes to examine the use of whole-body MRI treatment response assessment and cancer surveillance after curative treatments.
"At the moment, patients undergo surveillance scans after treatment for their cancer to look for recurrent disease," he said. "Currently we use conventional tests such as CT. We do not know if surveillance of patients with whole-body MRI will be more effective both in terms of accuracy and cost-effectiveness that using standard scans."
The group is working on setting up trials of staging whole-body MRI in other primary cancer sites, notably the breast. Also, they are collaborating with Prof. Andrea Rockall and her colleagues at Imperial College London to see if machine-learning techniques can help radiologists interpret whole-body MRI datasets.