In a new article about the implications of leaving the European Union (EU) for U.K.-based radiologists and their patients, senior radiologists reveal how the financial, organizational, ethical, and socio-political changes associated with Brexit will affect medical imaging.
In "Post Brexit: Challenges and Opportunities for Radiology Beyond the European Union" published online in the British Journal of Radiology on 6 February, the authors discuss how services may be affected, and how the split will herald a new era for imaging within the National Health Service (NHS).
 Radiologists should collectively discuss the fallout of Brexit to put contingency plans in place, according to Dr. Thomas Booth, PhD.
Radiologists should collectively discuss the fallout of Brexit to put contingency plans in place, according to Dr. Thomas Booth, PhD.By discussing the potential consequences of Brexit on healthcare finance, working conditions, policies and regulations, research opportunities, and health innovation, contingency plans can be put in place, according to the paper's co-author Dr. Thomas Booth, PhD, consultant diagnostic and interventional neuroradiologist at King's College Hospital NHS Foundation Trust in London. In an email exchange with AuntMinnieEurope.com, he outlined key issues that radiologists in the U.K. should consider.
"Brexit is now a reality and we should look for opportunities to pursue healthcare improvement for our patients. The paper summarizes these issues and gives some lobbying recommendations for the U.K. imaging community to shape policy in order to optimize the outcomes," Booth noted. "There is nothing individual radiologists need to prepare for per se. However, collectively, radiologists -- and more importantly the radiology, medical, and academic leaders shaping imaging in the U.K.-- need to influence policy, which is in an evolving state."
The authors make detailed recommendations in the paper. Namely, the U.K. government should do the following:
- Enact healthcare and scientific workforce policy quickly to allow those in the EU to plan careers in the U.K.
- Guarantee European collaboration in matters associated with the Medicine and Healthcare Products Regulatory Agency (MHRA) as soon as possible, and to fund and facilitate MHRA and National Institute for Health and Care Excellence (NICE) integration
- Guarantee European collaboration in matters associated with U.K. medical research -- in a similar manner to the U.K. government's recent funding guarantee for projects currently financed under the EU's Horizon 2020 research scheme
"The General Medical Council, the Royal Colleges, particularly the Royal College of Radiologists, and patient groups as well as universities, research councils, and research charities need to lobby government unceasingly to enable such recommendations," Booth stated.
Foreign workers
Because Brexit was decided by the slim majority vote of 52% in favor, lead figures from the U.K. imaging community have issued dire warnings that the shortfall in current radiologist numbers will worsen.
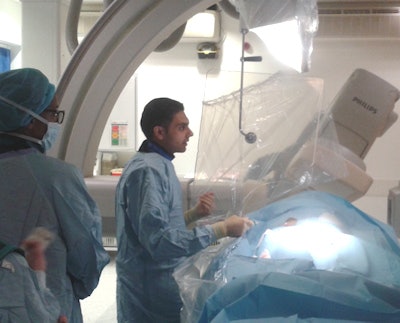 Will state-of the art imaging be threatened by Brexit's impact on radiological research? Here, interventional neuroradiology fellow, Dr. Ayman Qureshi and other interventional radiology staff, perform a procedure at King's College Hospital. All images courtesy of Dr. Thomas Booth, PhD.
Will state-of the art imaging be threatened by Brexit's impact on radiological research? Here, interventional neuroradiology fellow, Dr. Ayman Qureshi and other interventional radiology staff, perform a procedure at King's College Hospital. All images courtesy of Dr. Thomas Booth, PhD."It is premature to say there will be a greater shortage of radiologists in the U.K. following Brexit than there is currently. However, it is important that the U.K. government enacts healthcare workforce policy quickly to allow those in the EU to plan careers in the U.K.," he stressed.
The same was true for predicting whether the U.K. would become more or less attractive for radiology research. He pointed to the U.K. as a European research hub with a third of all EU-funded collaborations being led from the U.K., which gave it an advantageous position for establishing international relationships following formal separation from the EU. Again, much would depend on how quickly the government enacted scientific workforce policy and guaranteed European collaboration in medical research.
Authorization
Regulation was another area where fast action now would safeguard tomorrow's clinical and radiological studies, as well as vendor's markets.
Under current regulations, manufacturers only need to obtain a license in one member state to gain access to the EU-wide market. Inconsistency in standards within different countries meant that manufacturers might choose license authorization in countries with more lenient regulations.
 Radiologists at work in the radiology department, Ruskin Wing at King's College Hospital. Over 8% of all senior radiologists working in the U.K. gained their medical qualification from the EU, therefore the government must ensure workplace policy is adopted quickly to avoid an even larger shortfall in the U.K.'s imaging consultant numbers, according to the authors.
Radiologists at work in the radiology department, Ruskin Wing at King's College Hospital. Over 8% of all senior radiologists working in the U.K. gained their medical qualification from the EU, therefore the government must ensure workplace policy is adopted quickly to avoid an even larger shortfall in the U.K.'s imaging consultant numbers, according to the authors."The U.K. will now have a chance to review this legislation and establish its own national procedures, giving it an opportunity to enforce tougher standards, potentially benefitting patient safety," Booth told AuntMinnieEurope.com.
The paper pointed to the international influence enjoyed by the MHRA, and its readiness to develop policies allowing for this position to be maintained while cooperating more closely with NICE. According to the authors, international companies may still seek MHRA approval in the hope it will facilitate obtaining authorization in other countries.
However, the authors stated a separate U.K. authorization process may be unappealing due to additional costs precluding companies from accessing the British market, and delaying the availability of the newest technologies there.
Countering this argument is the fact that in 2014 the British medical technology market was the third largest in the EU, with an estimated worth of around 12 billion euros ($12.75 billion U.S.), they noted, which should ensure the U.K. authorization of devices continues. Furthermore, the U.K.'s low proportion of CT and MRI scanners per population compared with the EU average would continue to prove attractive to international vendors.
Positive change
The paper also highlights how health and safety regulations governing working conditions have to date been guided by the EU, including radiation protection regulations as well as the European Working Time Directive (EWTD). Outside the EU's internal market, the U.K. government will not be obliged to adhere to these, according to the authors.
"Since the EWTD was incorporated into the U.K. law in 1998, there has been an ongoing debate on the implications and effects that the EWTD has on medical training and professionalism. Concerns were raised over the resultant reduction in trainee clinical experience and exposure to learning opportunities as well as the fragmentation of patient care and its potential impact on patient safety. Brexit can be seen as an opportunity to introduce a change in working policies more suited to the NHS," they noted.