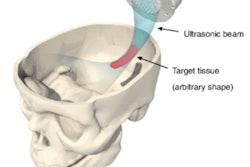
Brain diseases are notoriously difficult to treat due to the blood-brain barrier (BBB), a protective layer of cells limiting the delivery of most drugs into the brain. One promising approach for BBB disruption combines pulsed ultrasound with injected microbubbles, which vibrate in response to these sound waves, to temporarily open the BBB.
CarThera, a French start-up based at the Brain and Spine Institute at Pitié-Salpêtrière Hospital, is developing the SonoCloud device, an implantable ultrasound transducer that is fixed to the skull. The device has no internal energy source making it MRI-compatible, and is instead powered externally via a transcutaneous needle that's only connected during treatment sessions.
Alexandre Carpentier, CarThera's founder, inventor of SonoCloud, and a neurosurgeon at Pitié-Salpêtrière Hospital, and collaborators, have now tested the device in patients with recurrent glioblastoma, an aggressive and difficult to treat brain tumor. Preliminary results indicate the device can safely disrupt the BBB and boost the amount of drug delivered to the brain (Science Translational Medicine, Vol. 8:343, pp. 343re2).
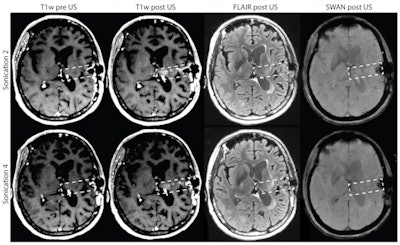 MR images from patient 15, who had a grade 3 BBB disruption in four of four sessions, show no evidence of tumor progression and a slight reduction in the fluid-attenuated inversion recovery (FLAIR) signal after four months on the study. Credit: A Carpentier et al, Science Translational Medicine (2016).
MR images from patient 15, who had a grade 3 BBB disruption in four of four sessions, show no evidence of tumor progression and a slight reduction in the fluid-attenuated inversion recovery (FLAIR) signal after four months on the study. Credit: A Carpentier et al, Science Translational Medicine (2016)."The blood-brain barrier is one of the last major frontiers of neuroscience," Carpentier said. "The publication of the trial results in one of the most prestigious U.S. scientific journals is a major acknowledgment of this medical first."
Preliminary success
The researchers implanted SonoCloud devices within the skulls of 15 patients, in areas overlying the tumors. In a dose-escalating phase 1/2a trial, the BBB of each patient was transiently disrupted at monthly treatment sessions using pulsed ultrasound and systemically injected microbubbles.
The first three patients were treated at an acoustic pressure of 0.5 MPa. Patients in the second cohort started at 0.65 MPa, with intrapatient dose escalation at subsequent sonications up to 0.95 MPa. Patients in the third, fourth, and fifth cohorts started at acoustic pressures of 0.8, 0.95, and 1.1 MPa, respectively, with dose escalation up to 1.1 MPa. In total, 41 sonications were performed in the 15 patients. All sonication cycles were followed by systemic infusion of carboplatin.
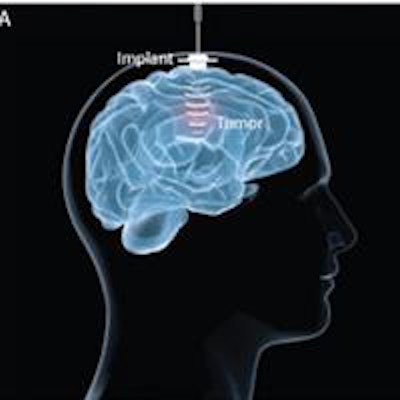
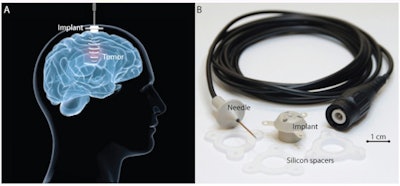 The implantable ultrasound device. (A) The device is fixed in the skull bone and connected to an external power supply by a transdermal needle connection during activation. (B) The implant comprises an ultrasound transducer encased in a biocompatible housing. The implant is passive and is connected by a transdermal needle to an external radiofrequency generator at each treatment to disrupt the BBB. Credit: A Carpentier et al, Science Translational Medicine (2016).
The implantable ultrasound device. (A) The device is fixed in the skull bone and connected to an external power supply by a transdermal needle connection during activation. (B) The implant comprises an ultrasound transducer encased in a biocompatible housing. The implant is passive and is connected by a transdermal needle to an external radiofrequency generator at each treatment to disrupt the BBB. Credit: A Carpentier et al, Science Translational Medicine (2016).Contrast-enhanced MR images revealed that no BBB disruption was achieved in patients receiving treatments at acoustic pressures of 0.5 or 0.65 MPa. Overall, BBB disruption was observed in 28 of the 41 sonications -- eight of 11 sonications at 0.8 MPa, six of seven sonications at 0.95 MPa, and all 14 sonications at 1.1 MPa -- without detectable adverse effects.
The trial is still ongoing, as the maximum tolerated ultrasound dose has not been reached. However, data to date are sufficient to infer that transient BBB disruption with the SonoCloud system can be achieved with a threshold pressure dose of 0.8 MPa. Preliminary findings indicate the approach is safe and well tolerated in patients with recurrent glioblastoma and has the potential to optimize chemotherapy delivery in the brain.
The researchers note that, although the trial's primary objective was not to assess treatment efficacy, in nine patients with confirmed BBB disruption, the region encompassed by the ultrasound field had no detected tumor progression on MRI. If efficacy holds up in further trials, the technique may offer a new treatment strategy for patients with brain cancer and potentially also neurodegenerative disorders.
 Alexandre Carpentier holding the SonoCloud implant. Credit: © G Dautricourt.
Alexandre Carpentier holding the SonoCloud implant. Credit: © G Dautricourt."The publication of this scientific paper supports the SonoCloud concept. It will allow us to start working on subsequent clinical developments with a view to marketing the device," said CarThera's CEO Frédéric Sottilini. "We plan to raise money in 2017 to fund a large-scale phase 2b/3 clinical trial in 200 patients, with centers in Europe and the U.S. SonoCloud could be commercially available in 2020 for use in recurrent glioblastoma, with CE Marking and FDA approval obtained in the meantime. At the same time, the company will carry out exploratory studies in other indications, including Alzheimer's disease."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.