A multidisciplinary team of U.K. experts has produced a comprehensive guide to help determine when the clinical use of PET/CT is justified. The 86-page document can be downloaded free of charge.
"These guidelines mark the 30th anniversary of clinical PET in the U.K.," explained lead author Prof. Sabina Dizdarevic, chair of the Intercollegiate Standing Committee on Nuclear Medicine (ICSCNM). "PET-CT has become a key multimodality molecular imaging technique in the assessment of a wide range of medical conditions."
Now in its fourth edition, the 2022 document provides updated indications with key references underpinning the use of FDG and non-FDG PET/CT tracers in malignant and nonmalignant diseases in clinical practice. It was compiled by a team of nuclear medicine physicians, radionuclide radiologists, and oncologists, noted Dizdarevic, who is the principal lead consultant in imaging and nuclear medicine at the Brighton and Sussex University Hospitals NHS Trust and PET/CT lead at the Clinical Imaging Science Centre.
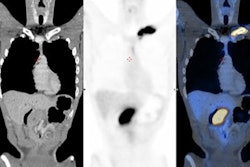
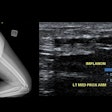
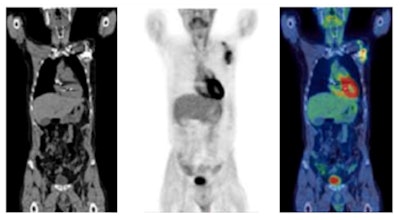 F-18 FDG PET/CT in the assessment of an infected cardiac pacemaker. (All figures courtesy of RCR)
F-18 FDG PET/CT in the assessment of an infected cardiac pacemaker. (All figures courtesy of RCR)The guidelines comprise an up-to-date summary of relevant indications for the use of PET/CT, where there is good evidence that patients will benefit from improved disease assessment, resulting in altered management and improved outcomes, according to the authors.
The indications are divided into oncological and nononcological applications followed by body area/system. This list is not exhaustive, and there are cases where PET/CT may be helpful in patients who have equivocal or definite abnormalities on other imaging where PET/CT may alter the management strategy if found to be "positive" or "negative"; for example, radical or high-risk surgery, they added.
PET/CT would be appropriate in such patients at the discretion of the local Administration of Radioactive Substances Advisory Committee license holder as a problem-solving tool when other imaging modalities have been inconclusive.
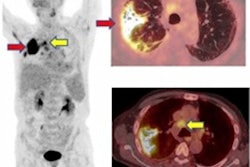
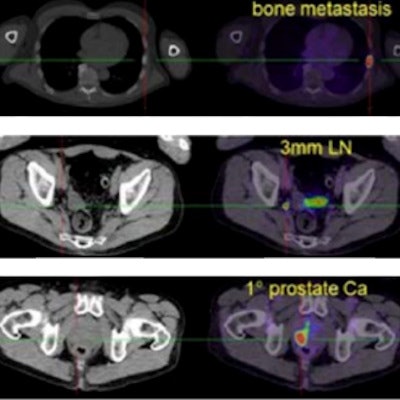
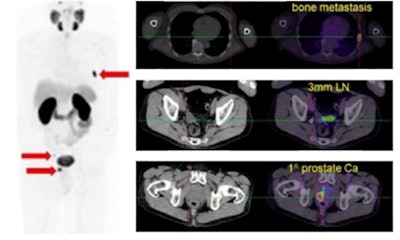 Gallium-68 (Ga-68)-labeled prostate-specific membrane antigen ligand PET/CT in prostate cancer.
Gallium-68 (Ga-68)-labeled prostate-specific membrane antigen ligand PET/CT in prostate cancer.The new document supersedes the previous "Evidence-Based Indications for the Use of PET-CT in the United Kingdom" guidelines published by the Royal College of Radiologists (RCR) in 2016. The document will be updated at regular intervals.
The 2022 report was prepared for the ICSCNM, the RCR, and the Royal College of Physicians by Dizdarevic, Prof. Andy Scarsbrook from Leeds, Prof. Sally Barrington from King's College London, and other specialists.
You can download it free of charge from the RCR website.