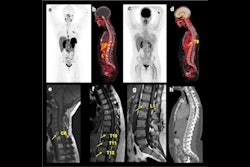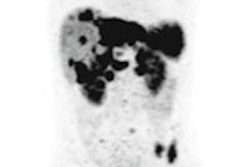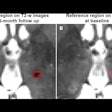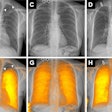
Award-winning researchers from a top London facility have demonstrated how lutetium-177 (Lu-177) peptide receptor radionuclide therapy (PRRT) is showing significant promise in the treatment of patients with inoperable or metastasized neuroendocrine tumors.
"Lu-177 PRRT is a safe and effective therapy, as evidenced by the NETTER-1 trial," noted Mubarik Arshad and his colleagues from the clinical imaging department at the Imperial College Healthcare National Health Service (NHS) Trust in London. "Lu-177 has been shown to prolong progression-free survival and overall survival compared to long-acting high-dose octreotide, with less than 10% significant hematologic toxicity and no renal toxicity when coadministered with positively charged amino acids."
This therapy is proving of particular value in patients with carcinoid tumors and gastroenteropancreatic neuroendocrine tumors (GEP-NETs), they added.
A pump infusion method of administration minimizes staff doses and results in minimal handling of the radiopeptide. Also, same-day discharge is proving successful at the Imperial, with individualized patient restrictions given based on the dose rate at discharge, the authors explained in a digital poster that won a certificate of merit at RSNA 2019 in Chicago.
Why a new therapy is needed
Many NETs overexpress somatostatin receptors (SSTRs), predominantly subtypes 2 and 5, and this overexpression of SSTRs acts as a target for both diagnostic and therapeutic purposes.
In the past, indium-111 diethylenetriamine pentaacetate (DTPA) octreotide, a gamma-emitting radiolabeled somatostatin analogue, was used both for the diagnosis and therapy of NETs, but with disappointing results in terms of tumor regression. This led to the development of the next generation of SSTR-mediated radionuclide therapy, which involves the use of a higher affinity somatostatin receptor type 2 analogue, in conjugation with high-energy β-emitters instead of γ-emitters, namely yttrium-90 (Y-90) and Lu-177.
"High γ emission from Lu-177 enables high-quality post-therapy tumor imaging and dosimetry, with lower whole-body retention and lower renal and bone marrow toxicity," Arshad and colleagues stated. "This amongst other factors has made Lu-177 the most widely available agent for PRRT."
The inclusion criteria are as follows:
- Positive SSTR imaging (indium-111 or gallium-68 peptides)
- Tumor differentiation: grade 1 or 2 NET; tumor proliferation: Ki67 ≤ 20%
- Life expectancy of more than 12 weeks
- Creatinine levels of ≤ 150 µmol/L or < 1.7 mg/dL; hemoglobin levels of ≥ 8.9 g/dL or 89 g/L; white cell count ≥ 2 g/dL; platelets ≥ 100 x109/L
- Karnofsky performance status ≥ 60 (requiring some help; can take care of most personal requirements)
Different infusion methods can be used for Lu-177 DOTATATE administration, and the method of choice often depends on the institution's experience and the relevant radioprotection measures in place, according to the authors. At the Imperial, they use the pump infusion method.
"Saline is pumped into the sealed vial of Lu-177 DOTATATE, causing the pressure in the vial to rise. The increasing pressure 'pushes' the Lu-177 DOTATATE out into the patient. The activity is constantly diluted during the administration -- the total volume in the vial remains constant," they pointed out. "Minimal handling is required. Residual Lu-177 DOTATATE is approximately 50 MBq. Staff doses are in the region of 3 µSv/administration."
They discharge patients between four and six hours after administration. A dose rate measurement is taken at 1 m from the patient torso immediately following administration and at discharge. Dose rates at discharge are typically 15 µSv to 40 µSv per hour.
Individualized patient restrictions are given based on the dose rate at discharge and an average effective half-life measured for an initial cohort of patients. Typical patient restrictions are to flush the toilet twice (for seven days), avoid close contact (more than one hour at less than 1 m) with children and pregnant women (6-10 days), avoid close contact (more than one hour at less than 1 m) with adults (1-4 days), and avoid sharing a bed with another person (5-9 days).
Looking to the future, the authors are convinced the following are of interest:
- Variability in the approach of delivering PRRT
- The combination of radionuclides (Y-90 and Lu-177) to optimize the therapeutic delivery of PRRT to lesions of differing sizes
- The use of radiosensitizing chemotherapy agents such as capecitabine to increase the efficacy of PRRT
- PRRT as a neoadjuvant or adjuvant intervention before or soon after surgery