Researchers at OncoRay in Dresden, Germany, have introduced a technique for more accurate proton therapy planning into routine clinical use. From April 2019 onward, they are implementing a novel, patient-specific method of proton range prediction based on dual-energy CT (DECT).
Acquiring images at two x-ray energies allows patients' tissue properties to be measured more directly, leading to more accurate estimates of proton range. As a result, treatment margins can be shrunk by as much as 40%, reducing radiation damage to healthy tissue surrounding the tumor and making proton therapy even more tolerable.
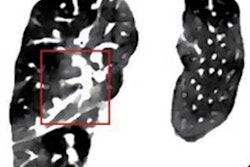
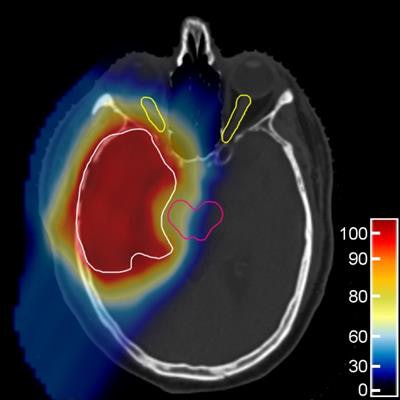
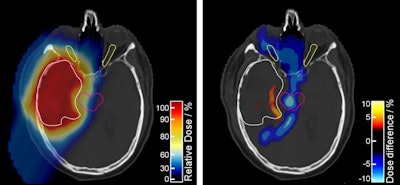 The proton treatment plan based on the DirectSPR method (left) results in substantially reduced dose to healthy tissue compared with a plan based on conventional lookup tables (dose difference map, right). Image courtesy of OncoRay/Christian Hahn, Nils Peters.
The proton treatment plan based on the DirectSPR method (left) results in substantially reduced dose to healthy tissue compared with a plan based on conventional lookup tables (dose difference map, right). Image courtesy of OncoRay/Christian Hahn, Nils Peters.The advantage of proton therapy over conventional x-ray radiotherapy lies in the narrowness of the particle's Bragg peak -- the distance over which it deposits its energy in a medium. Whereas the energy delivered by photons falls off gradually with distance, the dose distribution for charged particles such as protons rises sharply beyond a certain depth and then drops even more suddenly. The exact position and shape of the peak depend on the initial velocity of the particles in the beam and on the properties of the tissue along its trajectory.
This difference between the behavior of photons and charged particles means that proton dose patterns can be tailored to fit the shape of the tumor, sparing nearby tissue and organs. Unfortunately, it also means that translating planning images made using x-rays into dose maps for protons is not a simple operation.
Typically, medical physicists use a lookup table to convert x-ray radiopacity (CT number) into proton stopping power ratio (SPR), but this introduces into the process an uncertainty that can only be managed by expanding the volume of irradiated tissue -- otherwise, parts of the tumor might escape treatment. The unavoidable trade-off is that some surrounding healthy tissue is bound to be irradiated too, increasing the risk of damage to organs and triggering secondary cancers later in life.
Christian Richter, PhD, and colleagues from OncoRay introduced DECT for proton treatment planning at the University Proton Therapy Dresden (UPTD) in 2015. The technique yielded higher-quality images compared with single-energy scans, but standard lookup tables were still used for range prediction. Later, Richter and his translational research team used the growing database of clinical DECT scans to evaluate the clinical benefit of a more sophisticated, patient-specific method of range prediction.
 The project researchers (left to right): Christian Richter, PhD; Patrick Wohlfahrt, PhD; Christian Möhler, PhD; and Steffen Greilich, PhD. Image courtesy of Christian Richter, PhD.
The project researchers (left to right): Christian Richter, PhD; Patrick Wohlfahrt, PhD; Christian Möhler, PhD; and Steffen Greilich, PhD. Image courtesy of Christian Richter, PhD.Direct determination
When x-rays interact with tissue, photons can be scattered or absorbed. The strength of each effect depends on two material properties: relative electron density (RED) and effective atomic number (EAN). The contributions made by each of these attributes vary independently according to the photon energy, so capturing images at two different x-ray energies provides two sets of complementary data.
"Simplistically speaking, with one scan, we can determine one unknown. With two nonidentical scans we can determine two quantities," Richter said. This extra information allows RED and EAN, both important input parameters for calculating SPR, to be determined directly instead of relying on approximations.
Working with colleagues from the German Cancer Research Center (DKFZ) in Heidelberg, the OncoRay team validated the accuracy of the new approach, called DirectSPR, in a phantom and biological tissues and found that safety margins around the treatment volume could be reduced by 35% to 40%. The reduced margins achieved with DirectSPR are now applied clinically at UPTD. "The range accuracy, which has remained practically unchanged for more than 30 years, is thus for the first time significantly improved," Richter said.
Because the two scans are captured sequentially, DirectSPR is so far limited to static regions such as the head and pelvis; images taken of the chest would be too badly compromised by movement between acquisitions. Richter and colleagues have shown, though, that the technique is compatible with 4D CT -- in which patient motion is recorded as a video sequence -- so DirectSPR could be used in thoracic cancers soon.
For the time being, DirectSPR is available only in Dresden, where it has entered into routine operation. Although the method could in principle be employed using any DECT system, Richter and his team are collaborating with Siemens Healthineers to develop a commercial version for release this year, in which the DirectSPR calculation is integrated with the CT software.
Marric Stephens is a freelance science writer based in Bristol, U.K.
© IOP Publishing Limited. Republished with permission from Physics World, a website that helps scientists working in academic and industrial research stay up to date with the latest breakthroughs in physics and interdisciplinary science.