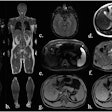
German researchers obtained positive results with a novel diagnostic and therapeutic (theranostic) approach for ductal pancreatic adenocarcinoma used with SPECT/CT, scintigraphy, and PET/CT. Their findings were published in the May issue of the Journal of Nuclear Medicine.
The study provides the first clinical evidence of the feasibility of treating ductal pancreatic adenocarcinoma with a DOTA-conjugated neurotensin receptor 1 (NTR1) antagonist, 3BP-227, labeled with the radioisotope lutetium-177 (Lu-177), according to a statement from the Society of Nuclear Medicine and Molecular Imaging (SNMMI). In preclinical research, Lu-177 3BP-227 was shown to significantly inhibit tumor growth.
For the study, six patients with confirmed metastatic ductal pancreatic adenocarcinoma received Lu-177 3BP-227 as salvage therapy. They underwent scintigraphy and SPECT/CT to assess tumor uptake and eligibility for treatment. Based on the patient's condition, FDG-PET/CT was performed eight to 12 weeks after therapy to determine treatment efficacy (JNM, May 2018, Vol. 59:5, pp. 809-814).
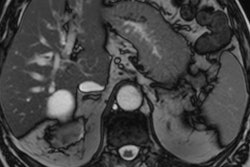
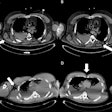
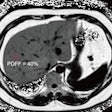
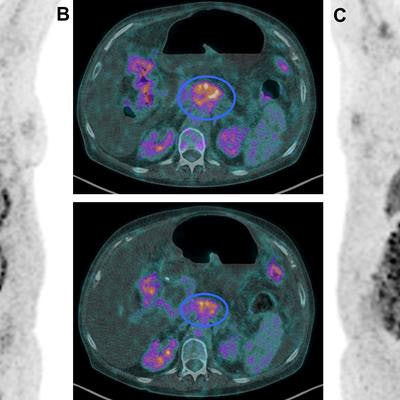
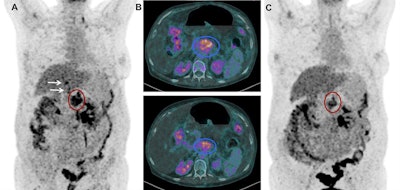 Images show FDG-PET and CT scans of a patient before and after treatment. A: FDG-PET before Lu-177 3BP-227 treatment (red oval: primary tumor; arrows: liver metastases). Upper panel of B: Axial CT section showing primary tumor (blue oval) before therapy. Lower panel of B: Axial CT section after three cycles of Lu-177 3BP-227. C: FDG-PET after three cycles of therapy. Images courtesy of Baum et al and JNM.
Images show FDG-PET and CT scans of a patient before and after treatment. A: FDG-PET before Lu-177 3BP-227 treatment (red oval: primary tumor; arrows: liver metastases). Upper panel of B: Axial CT section showing primary tumor (blue oval) before therapy. Lower panel of B: Axial CT section after three cycles of Lu-177 3BP-227. C: FDG-PET after three cycles of therapy. Images courtesy of Baum et al and JNM.Lu-177 3BP-227 was well-tolerated by the patients, the researchers found. In addition, one patient experienced significant improvement of symptoms and quality of life, surviving 13 months from diagnosis and 11 months from the start of therapy with Lu-177 3BP-227. The five-year survival rate for patients with this type of cancer is less than 5%.
"The research presented warrants further development of Lu-177 3BP-227, in order to provide patients with more effective treatment and less side effects than cytotoxic chemotherapy," said study co-author Christiane Smerling, PhD, head of nuclear medicine and imaging at 3B Pharmaceuticals in Berlin, in the SNMMI statement.