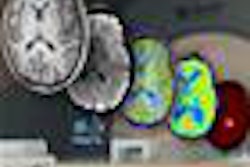
BERLIN - The marriage of PET and ultrahigh-field MRI offers a world of intriguing possibilities in neuroimaging -- including tools to analyze complex neural mechanisms and improve physicians' understanding of neurological disease processes, according to a Friday presentation at the Scientific Symposium on Ultrahigh Field Magnetic Resonance.
The challenges, too, are enormous but neuroscientists at the Jülich Research Center in Jülich, Germany -- among the first to install a PET camera inside a 9.4-tesla MRI magnet -- have solved most of the technical difficulties of combining the two modalities and are confident about the rest.
The challenges
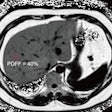
 MP-RAGE T1 image of a brain tumor combined with a PET image by F-fluoroethyl-tyrosine (FET). All images are courtesy of N. Jon Shah, PhD.
MP-RAGE T1 image of a brain tumor combined with a PET image by F-fluoroethyl-tyrosine (FET). All images are courtesy of N. Jon Shah, PhD.
Challenges arise with each modality separately and also when they're put together, said N. Jon Shah, PhD, managing director of the Center of Neuroscience and Medicine at Jülich during his presentation.
Ultrahigh-field MRI, for example, comes with signal homogeneity issues, specific absorption rate (SAR) issues, complications with shimming (adjusting signal homogeneity), and the problems with shorter T2 and T2* signals inherent in high-field MRI. Susceptibility problems are more acute, siting costs are high, and on top of it all, regulatory approval is a force to be reckoned with, Shah said.
"Once you've put PET inside the MR, one of the first things you lose is your radioactive source to do attenuation correction, so you've got to be able to use MRI to do that, especially if you want to ultimately go in the direction of quantitative PET," he said. "Then of course, PET is taking up space in your scanner, and it becomes somewhat confining in there."
Forget about photomultiplier tubes for PET, Shah said, you'll need solid-state detectors. And as the MRI magnet ring gets smaller with the addition of PET, you'll end up with wedge-shaped gaps between the detectors, he said. It's a challenge to get the arterial input apparatus in the ring, and you'll also need PET radiotracer production on site.
"One of the strengths of PET is that it's often considered the gold standard for many things because it's a quantitative technique, but when you put a PET scanner inside an MR machine that's one of the first things you lose," and it takes considerable fiddling to get it back, he added.
Of course, the 9.4-tesla whole-body MRI scanner (Siemens Healthcare) offers a number of advantages, including an improved signal-to-noise-ratio (SNR), better spectral resolution, and a stronger BOLD (blood-oxygen level-dependent) effect for functional MRI (fMRI), he said. But siting an instrument that weighs 870 tons takes a bit of effort.
There are profound susceptibility artifacts to deal with if you want to do functional brain imaging, in addition to wave effects in the form of high-frequency artifacts, and there are homogeneity issues to resolve around the B1 magnetic field.
You'll need a dedicated group to build coils and other hardware; the Jülich team has built an eight-channel sodium array and a 16-channel transmit-receive array, Shah said.
The opportunities
Fortunately, the possibilities of the hybrid combination are more impressive than the challenges, or at least they're more fun to talk about, Shah said.
First, it's easier to recruit volunteers for imaging studies when both PET and MRI can be acquired in a single scanning session, Shah said. Ultrahigh-field MR offers greatly improved spatial resolution, particularly for structural imaging, higher functional (BOLD) contrast, better image quality, and nonproton MRI and spectroscopy, Shah said.
"I think those are going to be huge opportunities for MRI at 9.4 tesla," he said. The improvement in contrast doesn't scale linearly with the increase in field strength but goes beyond it, he said. And image resolution is increased by a factor of 2.5 relative to a 1.5-tesla scanner.
For PET, MRI provides partial volume correction, attenuation correction, and motion correction via navigator echoes, Shah said.
"The beautiful structural images with MRI are pretty much automatically coregistered," he said. "In that you're doing simultaneous measurement, it's not that difficult to imagine that you're eventually going to splice navigator echo sequences into pretty much every sequence that you're running with MRI, and take the motion correction data with those measurements. Some of my colleagues think that navigator echo techniques won't have the same accuracy as motion detection with optical techniques, but I beg to differ." In fact, an upcoming study will demonstrate that navigator echo is superior to optical techniques, he said.
"The real opportunity of having hybrid MR-PET at 9.4 tesla is that you can start doing metabolic imaging in a very serious way," Shah said. "The clear tracer to use in PET will be FDG."
During the time the measurement is taking place, a patient can be given oxygen-17 gas to breathe, P31 MR spectroscopy can be administered to evaluate adenosine triphosphate (ATP), and finally, the entire imaging package can be bundled inside of a standard structural scan to determine exactly where in the brain relevant data is located, Shah said. These tools are all you need to do a serious exploration of brain metabolism, he said.
Novel paradigms for brain function are ripe for exploring with the new tools, he said. The process is challenged by the different time scales of the measurements in that PET takes longer than MRI. Researchers need to think about all of the imaging possibilities inherent in the systems, and come up with things to look for, Shah said.
Strong combination
While MRI has great spatial and temporal resolution, its specificity is on the low side, and it's not the gold standard for molecular imaging -- two areas where the addition of PET can be helpful. "Its specificity, based on the fact that you choose which tracer you're going to use, is excellent," Shah said.
On the molecular level, neurotransmission is driven by neurotransmitters or receptors or modulated by drugs -- the domain of PET, Shah said. Conversely, MRI works on the systemic level, depicting complex neural functions and providing localization and analysis of complex neural mechanisms at fMRI.
"One of the great opportunities at high-field MRI ... is the richness of the contrast that will have a huge impact on what you can do," he said. You don't have to do phase imaging -- you can just look at the magnitude images."
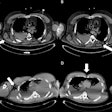
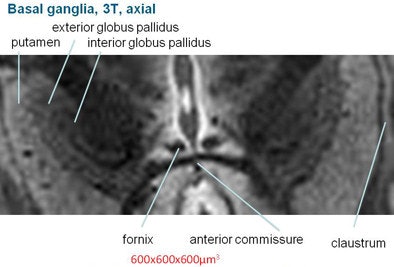 Basil ganglia image at 3 tesla (above) does not achieve the clarity, sharpness, and contrast of the image obtained at 9.4 tesla (below).
Basil ganglia image at 3 tesla (above) does not achieve the clarity, sharpness, and contrast of the image obtained at 9.4 tesla (below).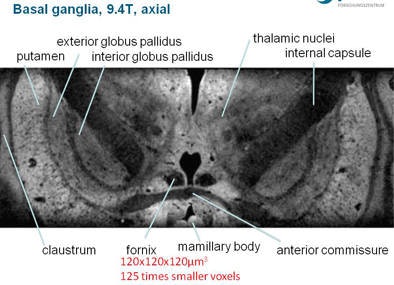
If you acquire the images with very high signal-to-noise ratios, you can selectively use filtering to get more specific information, Shah said.
In selective excitation (zoomed MRI) radiofrequency excitation of a region of interest can produce very high resolution of parts of the brain, such as the hippocampus, when the entire volume is not needed.
In a brain tumor imaging study, the use of hybrid scanning with F-fluoroethyl-tyrosene and ultrahigh-field MRI not only depicted the precise delineation of the tumor, it shows how it affects the surrounding brain regions, Shah said.
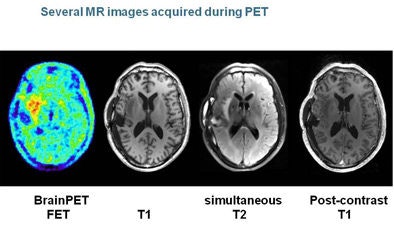
With ultrahigh-field MRI and PET neuroimaging "you have a positron flying around the magnetic field, and its motion is going to be circumscribed in two directions, and we should improve the resolution of the PET scan pretty much for free," he said.